Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 18 fevereiro 2025

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.

Workshop Report Circulation Research

Preclinical safety evaluation of biotechnology-derived
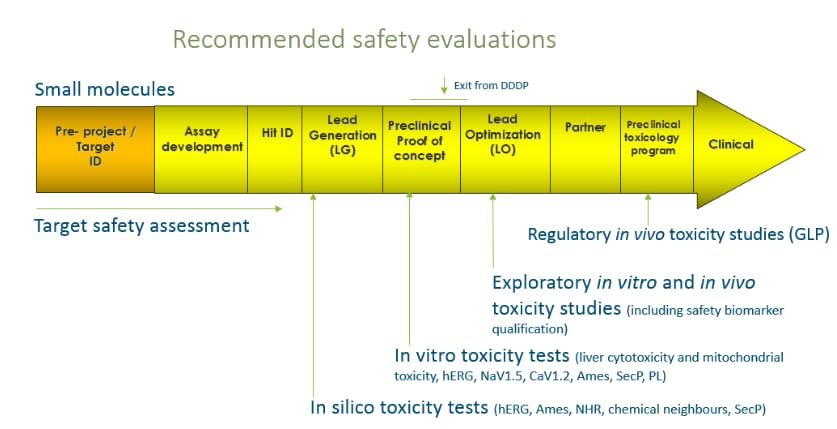
Target Product Profiling & Drug Safety Assessment - SciLifeLab
Cells, Free Full-Text

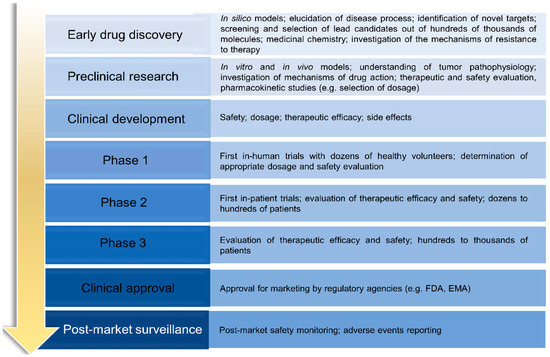
Drug discovery and development
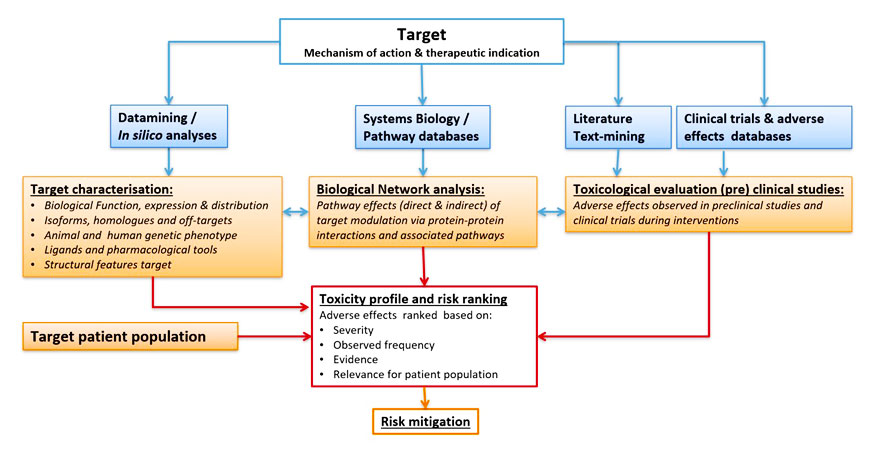
Target Profiling

Rational steps for drug discovery and development.

How drugs are discovered and developed - The Physiological Society
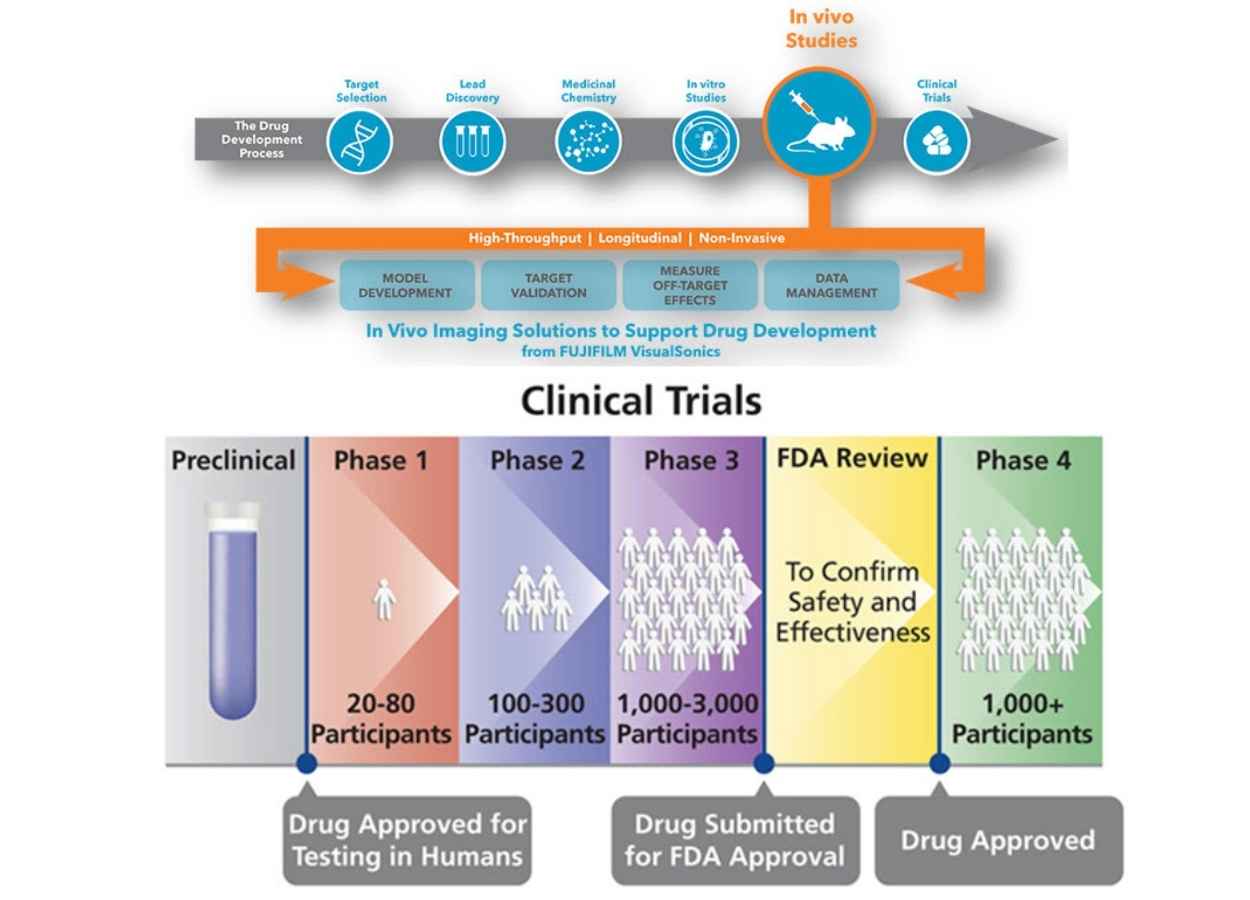
AN OVERVIEW OF NEW DRUG DISCOVERY AND DEVELOPMENT
Recomendado para você
-
 brain test Archives - Page 8 of 2818 fevereiro 2025
brain test Archives - Page 8 of 2818 fevereiro 2025 -
brain test level 411|Búsqueda de TikTok18 fevereiro 2025
-
 Brain Test Level 411 Walkthrough18 fevereiro 2025
Brain Test Level 411 Walkthrough18 fevereiro 2025 -
 Kunci Jawaban Brain Test Level 411 412 413 414 415 416 417: Sang18 fevereiro 2025
Kunci Jawaban Brain Test Level 411 412 413 414 415 416 417: Sang18 fevereiro 2025 -
CONSORT diagram. CCT conventional coagulation test, VHA18 fevereiro 2025
-
Hashimoto's 411 - If your iron is low, what is the best18 fevereiro 2025
-
 Classification of autism spectrum disorder based on sample entropy18 fevereiro 2025
Classification of autism spectrum disorder based on sample entropy18 fevereiro 2025 -
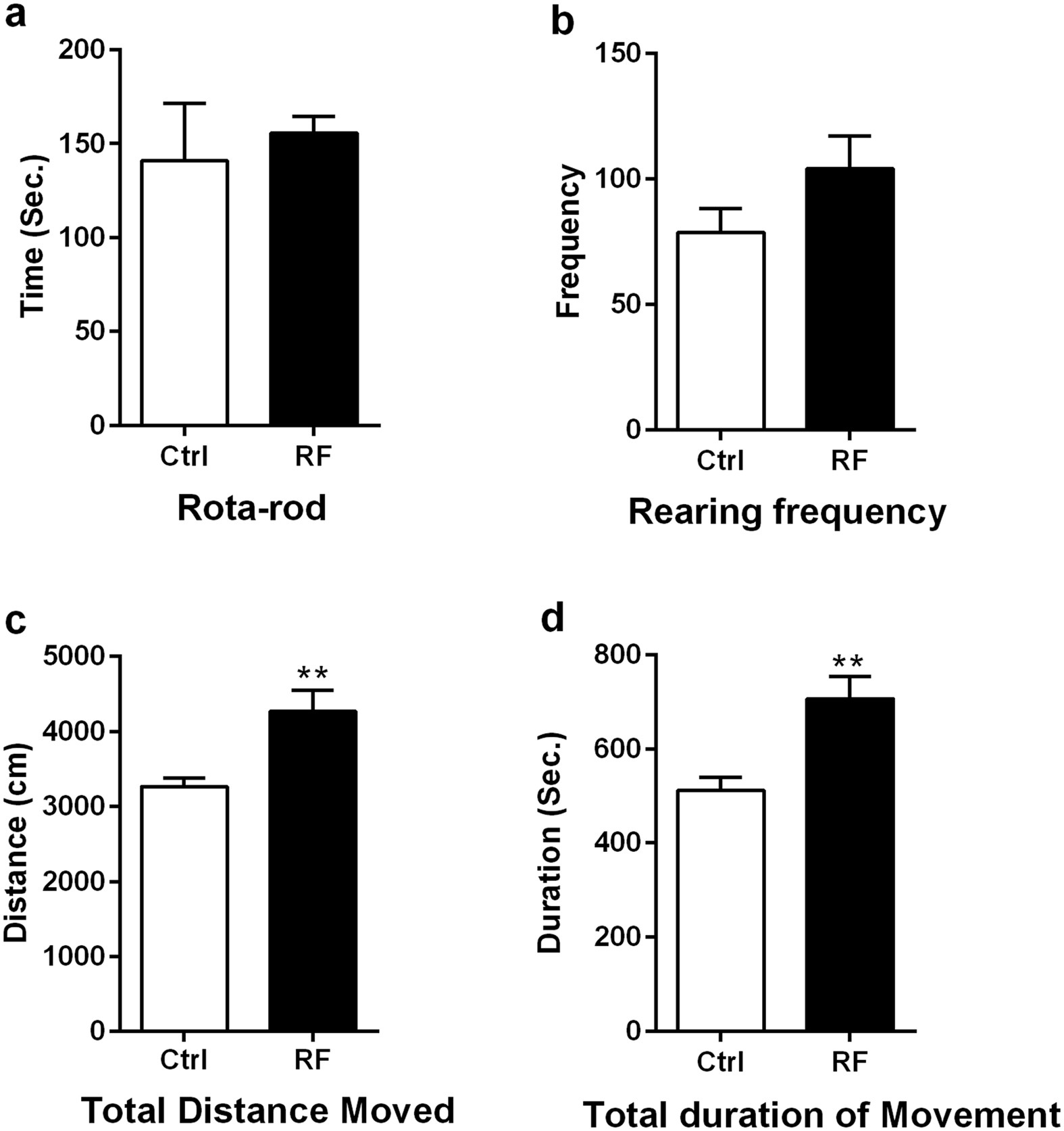 Long-term exposure to 835 MHz RF-EMF induces hyperactivity18 fevereiro 2025
Long-term exposure to 835 MHz RF-EMF induces hyperactivity18 fevereiro 2025 -
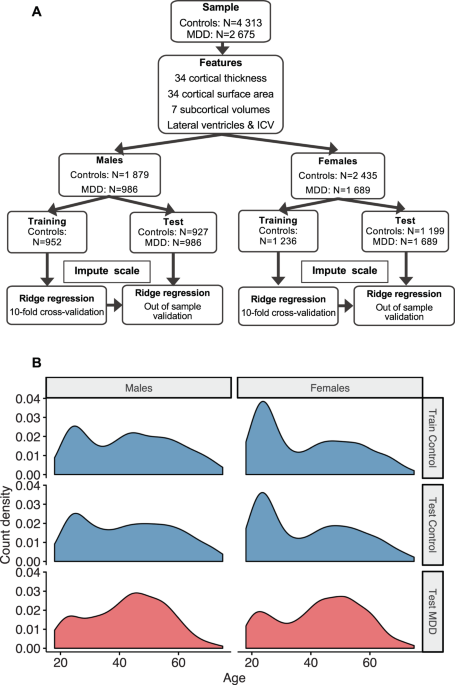 Brain aging in major depressive disorder: results from the ENIGMA18 fevereiro 2025
Brain aging in major depressive disorder: results from the ENIGMA18 fevereiro 2025 -
 Frequency-specific neuromodulation of local and distant18 fevereiro 2025
Frequency-specific neuromodulation of local and distant18 fevereiro 2025
você pode gostar
-
 Como Desenhar o YORIICHI TSUGIKUNI - Demon Slayer18 fevereiro 2025
Como Desenhar o YORIICHI TSUGIKUNI - Demon Slayer18 fevereiro 2025 -
2 player mahjong tutorial|TikTok Search18 fevereiro 2025
-
 soul eater the perfect edition Soul Eater: The Perfect Edition 0118 fevereiro 2025
soul eater the perfect edition Soul Eater: The Perfect Edition 0118 fevereiro 2025 -
TVOKids A Template - Sketchers United18 fevereiro 2025
-
 20 Und. UNO Jogo de Cartas - Deck de Cartas - Magazine Luiza18 fevereiro 2025
20 Und. UNO Jogo de Cartas - Deck de Cartas - Magazine Luiza18 fevereiro 2025 -
Random and crazy stuff - Dark Sonic - Wattpad18 fevereiro 2025
-
 MELHORES FOTOS DE ANIMES PARA COLOCAR NO PERFIL DO WHATSAPP18 fevereiro 2025
MELHORES FOTOS DE ANIMES PARA COLOCAR NO PERFIL DO WHATSAPP18 fevereiro 2025 -
 Pin em Atividades escola18 fevereiro 2025
Pin em Atividades escola18 fevereiro 2025 -
 Compre As Tartarugas Ninja - Boneco Donatello de 11cm do Filme18 fevereiro 2025
Compre As Tartarugas Ninja - Boneco Donatello de 11cm do Filme18 fevereiro 2025 -
![Osananajimi ga Zettai ni Makenai Love Comedy] Clear File [7] (Anime Toy) - HobbySearch Anime Goods Store](https://www.1999.co.jp/itbig75/10757712b_m.jpg) Osananajimi ga Zettai ni Makenai Love Comedy] Clear File [7] (Anime Toy) - HobbySearch Anime Goods Store18 fevereiro 2025
Osananajimi ga Zettai ni Makenai Love Comedy] Clear File [7] (Anime Toy) - HobbySearch Anime Goods Store18 fevereiro 2025




