FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Last updated 03 março 2025

Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw

Elias Jabbour MD Anderson Cancer Center

Here's who could be helped by new cancer drug — and when
Novartis challenges Pfizer with strong breast cancer drug data
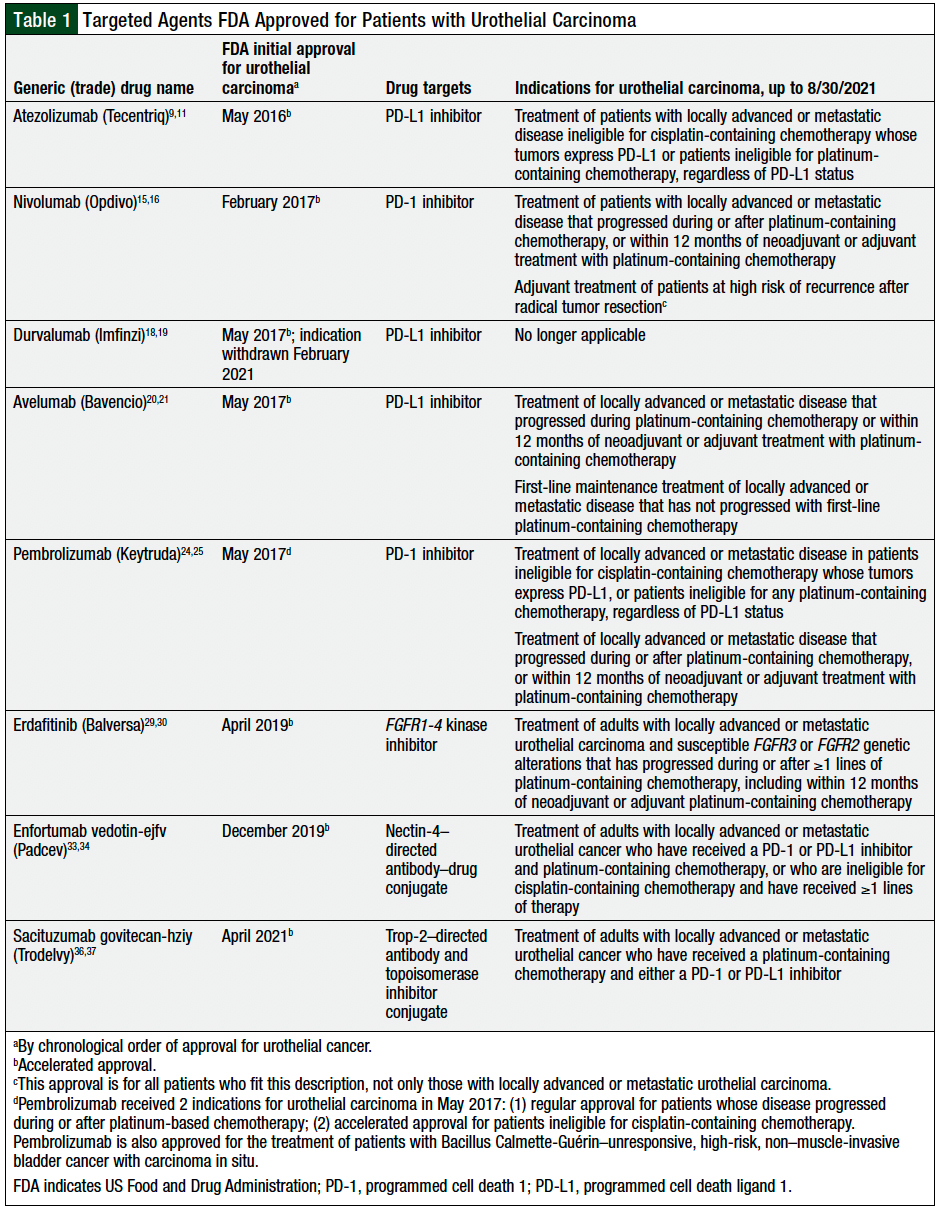
Recent Targeted Therapies Approved for the Treatment of Patients with Urothelial Carcinoma
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Druker-cancer-with-patient-631.jpg)
A Triumph in the War Against Cancer, Science

Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial - The Lancet Oncology

Hagop M. Kantarjian MD Anderson Cancer Center

Controversial Texas doctor Stanislaw Burzynski goes before disciplinary board

Stephen Hahn - Wikipedia

Texas judges clear Houston cancer doctor of most medical misconduct charges

Ashish M. Kamat MD Anderson Cancer Center
Recomendado para você
-
 Younan Nowzaradan - Age, Bio, Birthday, Family, Net Worth03 março 2025
Younan Nowzaradan - Age, Bio, Birthday, Family, Net Worth03 março 2025 -
 Dr now houston tx office visit from fan|TikTok Search03 março 2025
Dr now houston tx office visit from fan|TikTok Search03 março 2025 -
 Dr. Now MD on Instagram: “How y'all doing? #motivationmonday ShopDrNow.com fridge magnets! #drnowmd #shopdrnow #weight…03 março 2025
Dr. Now MD on Instagram: “How y'all doing? #motivationmonday ShopDrNow.com fridge magnets! #drnowmd #shopdrnow #weight…03 março 2025 -
 Everyone's favorite motivational weight doctor Dr. Now at your service!03 março 2025
Everyone's favorite motivational weight doctor Dr. Now at your service!03 março 2025 -
🚨New🚨🩺Dr. Joye Taylor-Houston Haematologist / Oncologist : Specializing in: ▪️Benign Haematology Anaemia Thrombocytopenia…03 março 2025
-
 Brian Allan Houston, MD in Charleston, SC, Specializes in: Cardiology - Heart Failure & Transplant03 março 2025
Brian Allan Houston, MD in Charleston, SC, Specializes in: Cardiology - Heart Failure & Transplant03 março 2025 -
 Patients of Dr. G.K. Ravichandran, TX03 março 2025
Patients of Dr. G.K. Ravichandran, TX03 março 2025 -
 Sarasota cancer survivor pays it forward, with blanket warmers for patients03 março 2025
Sarasota cancer survivor pays it forward, with blanket warmers for patients03 março 2025 -
 Robert H. Ball, MD Texas Children's Pavilion for Women03 março 2025
Robert H. Ball, MD Texas Children's Pavilion for Women03 março 2025 -
 What treatments are being used by hospitals to treat COVID?03 março 2025
What treatments are being used by hospitals to treat COVID?03 março 2025
você pode gostar
-
𝕱𝖗𝖊𝖞𝖆 𝕯𝖆𝖍𝖑𝖘𝖙𝖗𝖔𝖒 ✨ on Instagram: Amy Lee (AN: if u03 março 2025
-
 El final del paraíso de telemundo, el cast03 março 2025
El final del paraíso de telemundo, el cast03 março 2025 -
 Watashi Wa - “Like You Mean It” (Video) - SOUND IN THE SIGNALS03 março 2025
Watashi Wa - “Like You Mean It” (Video) - SOUND IN THE SIGNALS03 março 2025 -
 PlayStation Plus na grudzień 2023 to wyścigi LEGO i czyszczenie03 março 2025
PlayStation Plus na grudzień 2023 to wyścigi LEGO i czyszczenie03 março 2025 -
/cdn.vox-cdn.com/uploads/chorus_asset/file/23615157/ENUS_CyberpunkE_S1_Main_Horizontal_16x9_RGB_PRE.jpg) Watch the first trailer for the Cyberpunk: Edgerunners anime - The Verge03 março 2025
Watch the first trailer for the Cyberpunk: Edgerunners anime - The Verge03 março 2025 -
![Manga]Character heights taken from the official guidebook : r/VinlandSaga](https://i.imgur.com/I63CBdy.jpg) Manga]Character heights taken from the official guidebook : r/VinlandSaga03 março 2025
Manga]Character heights taken from the official guidebook : r/VinlandSaga03 março 2025 -
 Download DA REAL INSANE album songs: SAITAMA RAP SONG03 março 2025
Download DA REAL INSANE album songs: SAITAMA RAP SONG03 março 2025 -
 Fire Force Personagens de anime, Desenhos, Anime03 março 2025
Fire Force Personagens de anime, Desenhos, Anime03 março 2025 -
 Criar Puzzle de graça, Interacty03 março 2025
Criar Puzzle de graça, Interacty03 março 2025 -
 Re-L Mayer // Ergo Proxy by Hime-sOph on DeviantArt03 março 2025
Re-L Mayer // Ergo Proxy by Hime-sOph on DeviantArt03 março 2025

