FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 21 fevereiro 2025

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

For Alzheimer's Agitation, Promising News from Rexulti (Brexpiprazole)
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

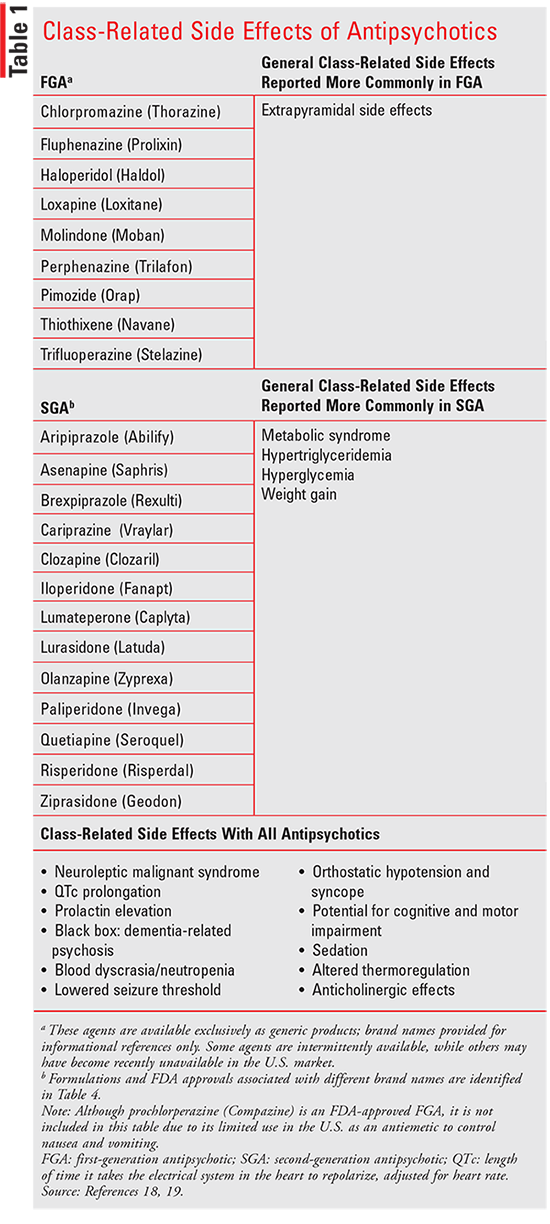
FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services
Rexulti (Brexpiprazole): Side Effects, Use for Depression, and More
Lesson: Assessing the Current Antipsychotics Landscape
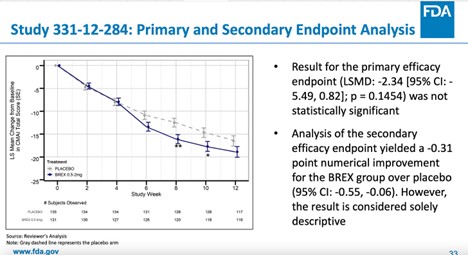
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA fast-tracked dementia drug with known harms, reporter says - Clinical Daily News - McKnight's Long-Term Care News

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services
Recomendado para você
-
 For Alzheimer's Agitation, Promising News from Rexulti (Brexpiprazole)21 fevereiro 2025
For Alzheimer's Agitation, Promising News from Rexulti (Brexpiprazole)21 fevereiro 2025 -
What you need to know about Rexulti (Brexpiprazole)21 fevereiro 2025
-
 REXULTI (brexpiprazole) Tablet21 fevereiro 2025
REXULTI (brexpiprazole) Tablet21 fevereiro 2025 -
 dtw Research, Inc on X: Throughout 2019, Rexulti produces the largest volume of marketing materials🔍📈 #lundbeck #otsuka #rexulti #brexpiprazole #depression #majordepressivedisorder #MDD #centralnervoussystem #CNS #antidepressants #yearendreview21 fevereiro 2025
dtw Research, Inc on X: Throughout 2019, Rexulti produces the largest volume of marketing materials🔍📈 #lundbeck #otsuka #rexulti #brexpiprazole #depression #majordepressivedisorder #MDD #centralnervoussystem #CNS #antidepressants #yearendreview21 fevereiro 2025 -
 Rexulti - Otsuka Pharmaceutical Co., Ltd.21 fevereiro 2025
Rexulti - Otsuka Pharmaceutical Co., Ltd.21 fevereiro 2025 -
 Abilify vs. Rexulti: Differences, similarities, and which is better for you21 fevereiro 2025
Abilify vs. Rexulti: Differences, similarities, and which is better for you21 fevereiro 2025 -
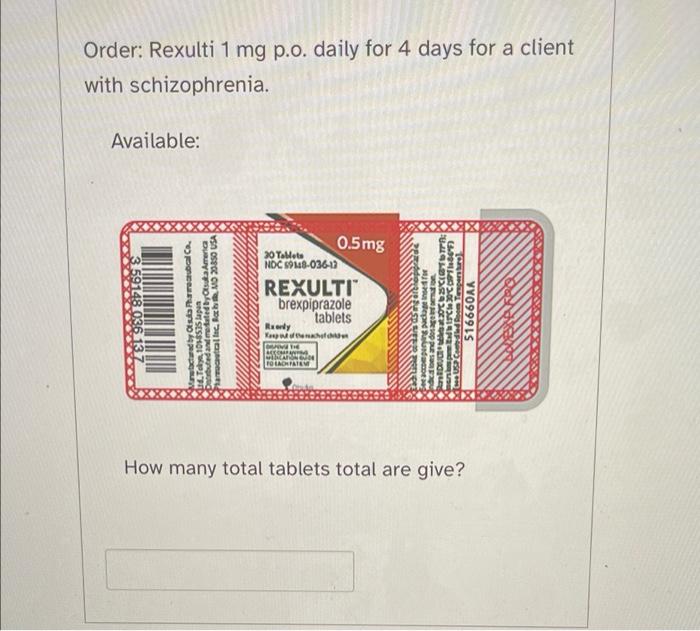
Solved Order: Rexulti 1 mg p.o. daily for 4 days for a21 fevereiro 2025
-
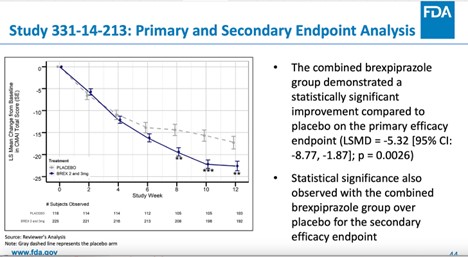
 Rexulti Works for Agitation in Alzheimer's, Despite Risks, Says FDA Staff21 fevereiro 2025
Rexulti Works for Agitation in Alzheimer's, Despite Risks, Says FDA Staff21 fevereiro 2025 -
 New Drug Product: REXULTI - MPR21 fevereiro 2025
New Drug Product: REXULTI - MPR21 fevereiro 2025 -
 Rexulti Patient Site - Once Daily Pharma21 fevereiro 2025
Rexulti Patient Site - Once Daily Pharma21 fevereiro 2025
você pode gostar
-
 The Last of Us Part 1 review21 fevereiro 2025
The Last of Us Part 1 review21 fevereiro 2025 -
 MORTAL KOMBAT Fatalities That Are Now Brutalities21 fevereiro 2025
MORTAL KOMBAT Fatalities That Are Now Brutalities21 fevereiro 2025 -
 Christmas With Nat King Cole Dean Martin and Bing Crosby - in 202321 fevereiro 2025
Christmas With Nat King Cole Dean Martin and Bing Crosby - in 202321 fevereiro 2025 -
 ☊ Clash Royale Soundboard21 fevereiro 2025
☊ Clash Royale Soundboard21 fevereiro 2025 -
 Os Melhores Animes Dublados de 2019 - Anime United21 fevereiro 2025
Os Melhores Animes Dublados de 2019 - Anime United21 fevereiro 2025 -
 Claire Forlani Poster #77029 Online21 fevereiro 2025
Claire Forlani Poster #77029 Online21 fevereiro 2025 -
 A Wooden Imperial Palace that Defies History: Forbidden City21 fevereiro 2025
A Wooden Imperial Palace that Defies History: Forbidden City21 fevereiro 2025 -
 How to Decorate Cement Tealight Candle Holder Using Dot Mandala21 fevereiro 2025
How to Decorate Cement Tealight Candle Holder Using Dot Mandala21 fevereiro 2025 -
 Matemática – Multiplicação e características das pirâmides – Conexão Escola SME21 fevereiro 2025
Matemática – Multiplicação e características das pirâmides – Conexão Escola SME21 fevereiro 2025 -
 Tudo sobre os Jinchuurikis de Naruto: bijus e seus portadores21 fevereiro 2025
Tudo sobre os Jinchuurikis de Naruto: bijus e seus portadores21 fevereiro 2025