Molecular Mechanisms in Genetic Aortopathy–Signaling Pathways and Potential Interventions
Por um escritor misterioso
Last updated 20 janeiro 2025
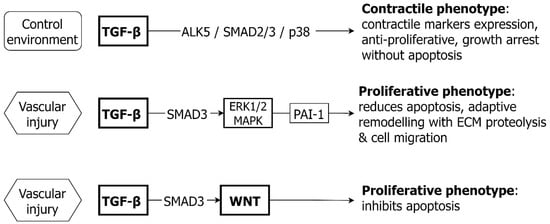
Thoracic aortic disease affects people of all ages and the majority of those aged <60 years have an underlying genetic cause. There is presently no effective medical therapy for thoracic aneurysm and surgery remains the principal intervention. Unlike abdominal aortic aneurysm, for which the inflammatory/atherosclerotic pathogenesis is well established, the mechanism of thoracic aneurysm is less understood. This paper examines the key cell signaling systems responsible for the growth and development of the aorta, homeostasis of endothelial and vascular smooth muscle cells and interactions between pathways. The evidence supporting a role for individual signaling pathways in pathogenesis of thoracic aortic aneurysm is examined and potential novel therapeutic approaches are reviewed. Several key signaling pathways, notably TGF-β, WNT, NOTCH, PI3K/AKT and ANGII contribute to growth, proliferation, cell phenotype and survival for both vascular smooth muscle and endothelial cells. There is crosstalk between pathways, and between vascular smooth muscle and endothelial cells, with both synergistic and antagonistic interactions. A common feature of the activation of each is response to injury or abnormal cell stress. Considerable experimental evidence supports a contribution of each of these pathways to aneurysm formation. Although human information is less, there is sufficient data to implicate each pathway in the pathogenesis of human thoracic aneurysm. As some pathways i.e., WNT and NOTCH, play key roles in tissue growth and organogenesis in early life, it is possible that dysregulation of these pathways results in an abnormal aortic architecture even in infancy, thereby setting the stage for aneurysm development in later life. Given the fine tuning of these signaling systems, functional polymorphisms in key signaling elements may set up a future risk of thoracic aneurysm. Multiple novel therapeutic agents have been developed, targeting cell signaling pathways, predominantly in cancer medicine. Future investigations addressing cell specific targeting, reduced toxicity and also less intense treatment effects may hold promise for effective new medical treatments of thoracic aortic aneurysm.
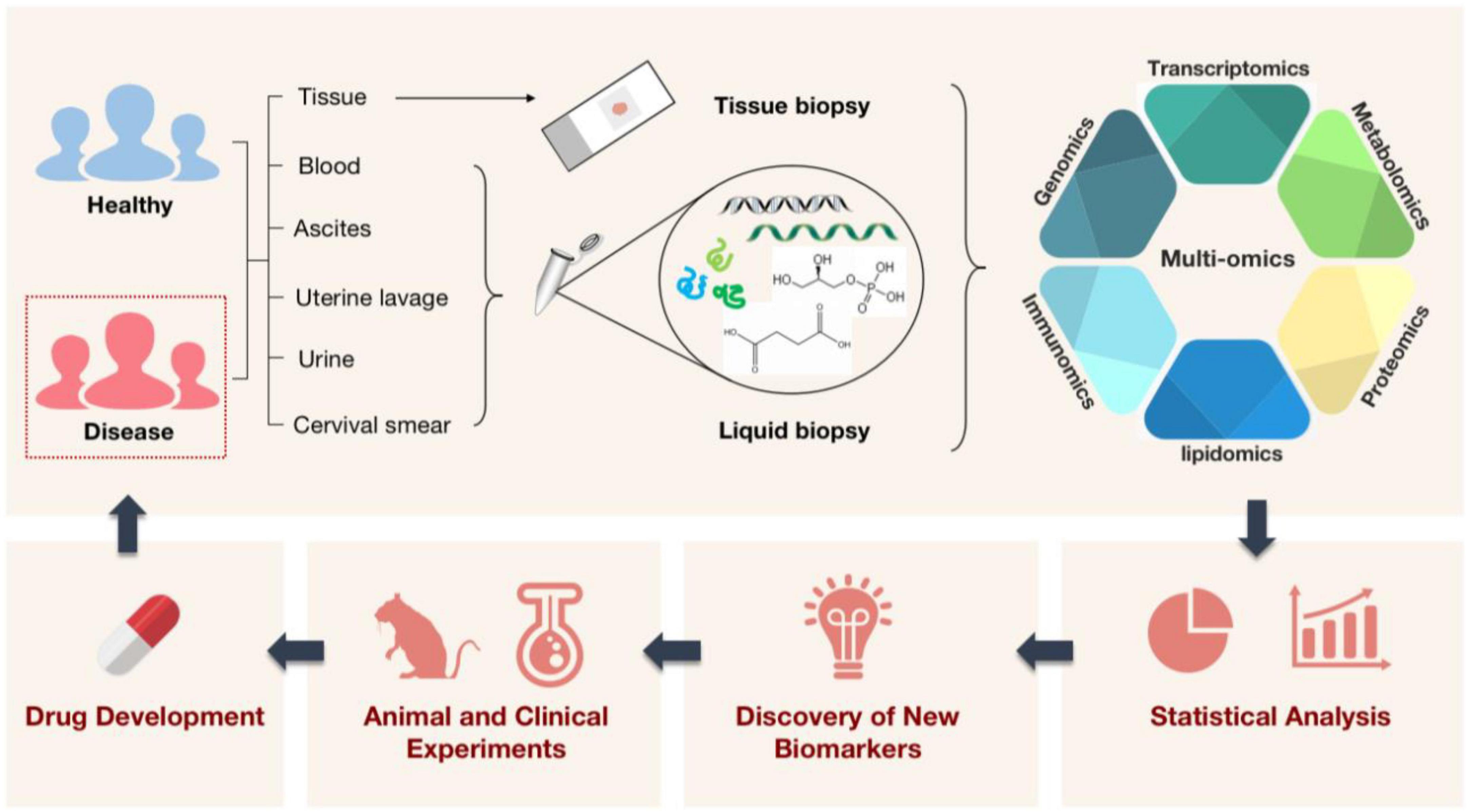
Frontiers Applying multi-omics techniques to the discovery of biomarkers for acute aortic dissection

The genetic basis of thoracic aortic disease: The future of aneurysm classification? - ScienceDirect

Recent Advances in Understanding the Molecular Pathophysiology of Angiotensin II Receptors: Lessons From Cell-Selective Receptor Deletion in Mice - Canadian Journal of Cardiology

Wnt signaling in cardiovascular physiology

Summary of evidence for signaling pathways in pathogenesis of thoracic

Chondrodysplasias and Aneurysmal Thoracic Aortopathy: An Emerging Tale of Molecular Intersection: Trends in Molecular Medicine
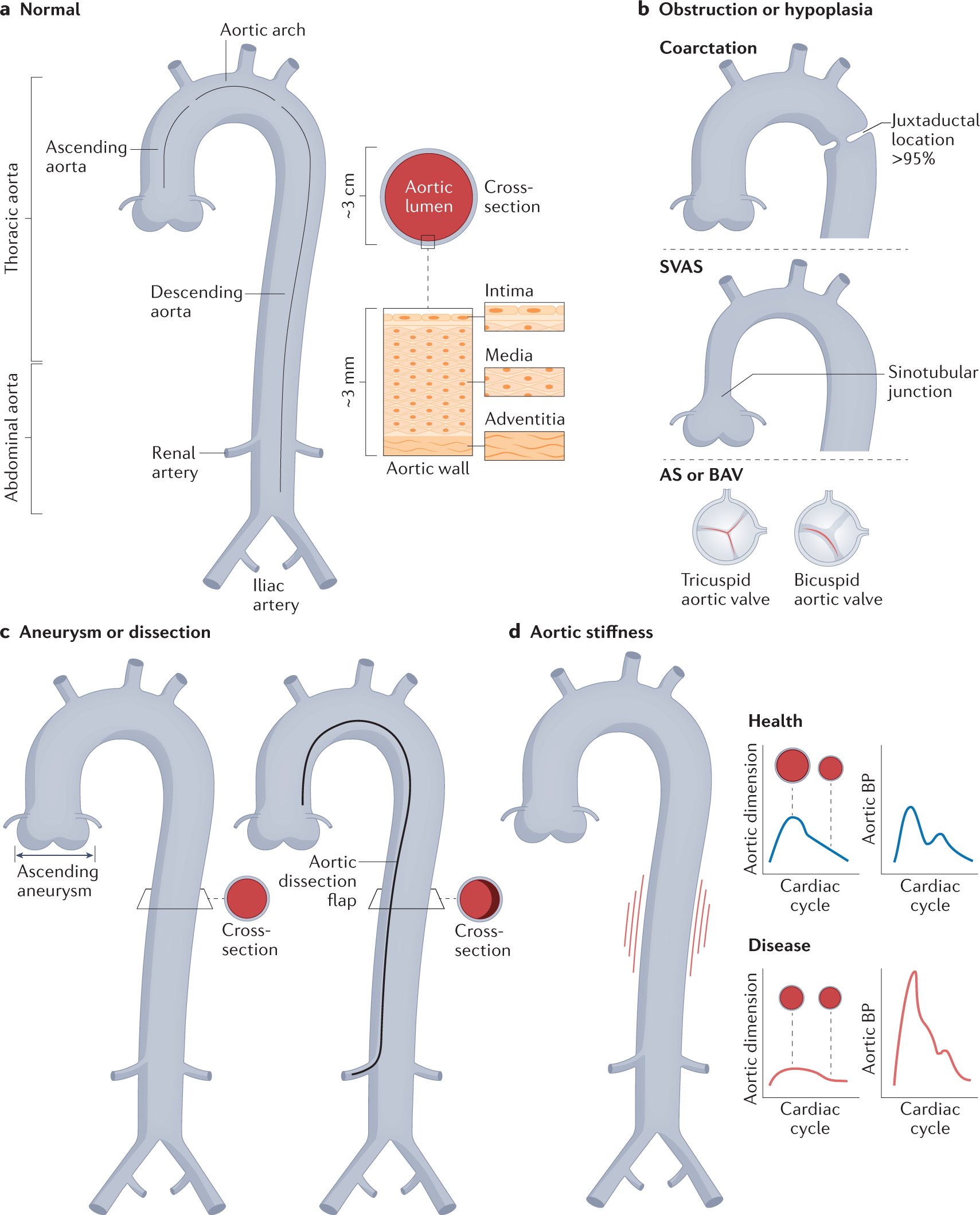
Genetics and mechanisms of thoracic aortic disease

The genetics and biomechanics of thoracic aortic diseases in: Vascular Biology Volume 1 Issue 1 (2019)

IJMS, Free Full-Text
Recomendado para você
-
 Linn da Quebrada sofre transfobia e é chamada de 'troço' em podcast20 janeiro 2025
Linn da Quebrada sofre transfobia e é chamada de 'troço' em podcast20 janeiro 2025 -
 Aniversário: produção de carvão acompanha várias gerações em Linha Nova - Jornal O Diário20 janeiro 2025
Aniversário: produção de carvão acompanha várias gerações em Linha Nova - Jornal O Diário20 janeiro 2025 -
 Tô com hem0rr0ida (058) Saco Cheio Podcast com Arthur Petry20 janeiro 2025
Tô com hem0rr0ida (058) Saco Cheio Podcast com Arthur Petry20 janeiro 2025 -
![ARTHUR PETRY E VILELA - [MELHORES MOMENTOS] - PODIHHCAST](https://i.ytimg.com/vi/2_7AeH61hSU/maxresdefault.jpg) ARTHUR PETRY E VILELA - [MELHORES MOMENTOS] - PODIHHCAST20 janeiro 2025
ARTHUR PETRY E VILELA - [MELHORES MOMENTOS] - PODIHHCAST20 janeiro 2025 -
 ARTHUR PETRY fala sobre um mundo Gay20 janeiro 2025
ARTHUR PETRY fala sobre um mundo Gay20 janeiro 2025 -
 2023 Archives - Teatro Renassaince20 janeiro 2025
2023 Archives - Teatro Renassaince20 janeiro 2025 -
 Audiobook Todo Dia Podcast20 janeiro 2025
Audiobook Todo Dia Podcast20 janeiro 2025 -
Sabe uma coisa que eu odeio? MOLHERES” #piada #humortiktok #futebol #20 janeiro 2025
-
CortesMágicosTikTok (@cortesmagicostiktok)20 janeiro 2025
-
 Sophie conversa a sós com o pai de Iuri20 janeiro 2025
Sophie conversa a sós com o pai de Iuri20 janeiro 2025
você pode gostar
-
 ESTOU MUITO PERTO de ENTRAR NO TOP GLOBAL! ANIME WARRIORS 220 janeiro 2025
ESTOU MUITO PERTO de ENTRAR NO TOP GLOBAL! ANIME WARRIORS 220 janeiro 2025 -
 One Piece Chapter 1065 Recap & Spoilers: Six Vegapunks20 janeiro 2025
One Piece Chapter 1065 Recap & Spoilers: Six Vegapunks20 janeiro 2025 -
 Torneio de fim de ano Tênis de Campo TCPP – Tenis Clube20 janeiro 2025
Torneio de fim de ano Tênis de Campo TCPP – Tenis Clube20 janeiro 2025 -
U13 - Enemigos Públicos nº1 - Capítulo 74, Página 1700 - DBMultiverse20 janeiro 2025
-
 Jogo Xbox One E Series X Crash Team Rumble Deluxe Fisico20 janeiro 2025
Jogo Xbox One E Series X Crash Team Rumble Deluxe Fisico20 janeiro 2025 -
 RUMOR: Roteiro vazado de Deadpool 3 se conecta com Loki - Nova Era Geek20 janeiro 2025
RUMOR: Roteiro vazado de Deadpool 3 se conecta com Loki - Nova Era Geek20 janeiro 2025 -
 Quanzhi Gaoshou (The King's Avatar) - Chapter 7020 janeiro 2025
Quanzhi Gaoshou (The King's Avatar) - Chapter 7020 janeiro 2025 -
 Trying to ensure the spirit of the early games remains intact20 janeiro 2025
Trying to ensure the spirit of the early games remains intact20 janeiro 2025 -
 2023 U.S. Chess Champions: Grandmaster Fabiano Caruana Defends Title to Become a Three-Time U.S. Champion; International Master Carissa Yip Wins Second Title in Women's Division20 janeiro 2025
2023 U.S. Chess Champions: Grandmaster Fabiano Caruana Defends Title to Become a Three-Time U.S. Champion; International Master Carissa Yip Wins Second Title in Women's Division20 janeiro 2025 -
 Fall Color on Display at Alabama's State Parks20 janeiro 2025
Fall Color on Display at Alabama's State Parks20 janeiro 2025

