Statistics in Medicine — Reporting of Subgroup Analyses in
Por um escritor misterioso
Last updated 18 dezembro 2024


Randomized controlled trial - Wikipedia

A critical review of graphics for subgroup analyses in clinical trials - Ballarini - 2020 - Pharmaceutical Statistics - Wiley Online Library
Subgroup Analysis - Statistics How To

Statistical Analysis Plan: What is it & How to Develop it
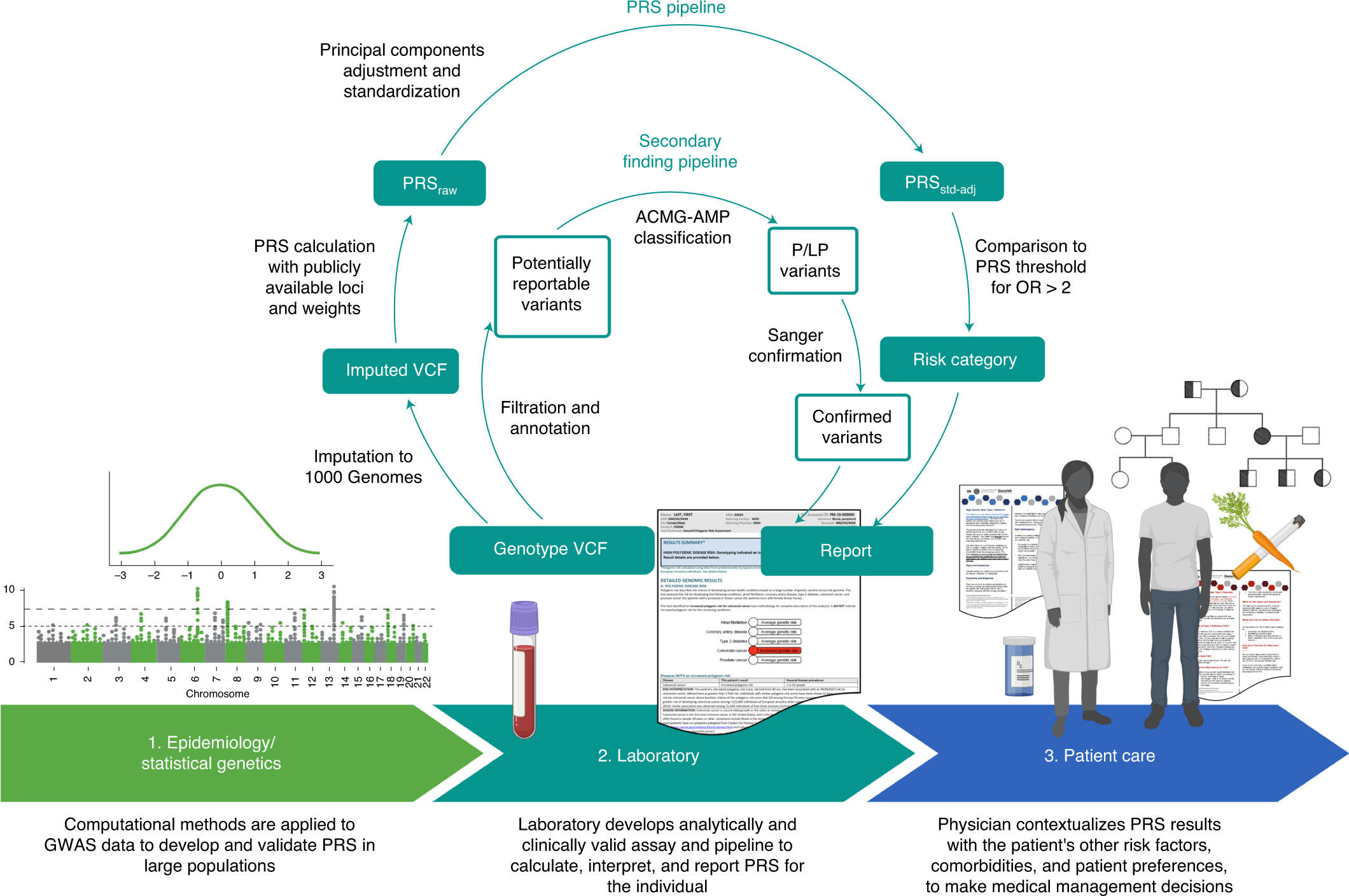
Development of a clinical polygenic risk score assay and reporting workflow

Statistics in Medicine — Reporting of Subgroup Analyses in Clinical Trials

Subgroup analyses. Deaths and tests for interaction by subgroup. HRs

PDF) Statistics in Medicine — Reporting of Subgroup Analyses in Clinical Trials

Applying Propensity Score Methods in Clinical Research in Neurology

Understanding Clinical Data Analysis: Learning Statistical Principles from Published Clinical Research

The lack of statistical power of subgroup analyses in meta-analyses: a cautionary note, Epidemiology and Psychiatric Sciences
Improving the Transparency of Prognosis Research: The Role of Reporting, Data Sharing, Registration, and Protocols

A comprehensive analysis of the efficacy and safety of COVID-19 vaccines: Molecular Therapy
Recomendado para você
-
 Introduction to Meta-Analyses18 dezembro 2024
Introduction to Meta-Analyses18 dezembro 2024 -
 Sample Analyses · SENAITE18 dezembro 2024
Sample Analyses · SENAITE18 dezembro 2024 -
 Announcing the Upcoming Release of the 2022 NBCOT Practice Analyses18 dezembro 2024
Announcing the Upcoming Release of the 2022 NBCOT Practice Analyses18 dezembro 2024 -
 Statistical Analyses for Language Assessment Book18 dezembro 2024
Statistical Analyses for Language Assessment Book18 dezembro 2024 -
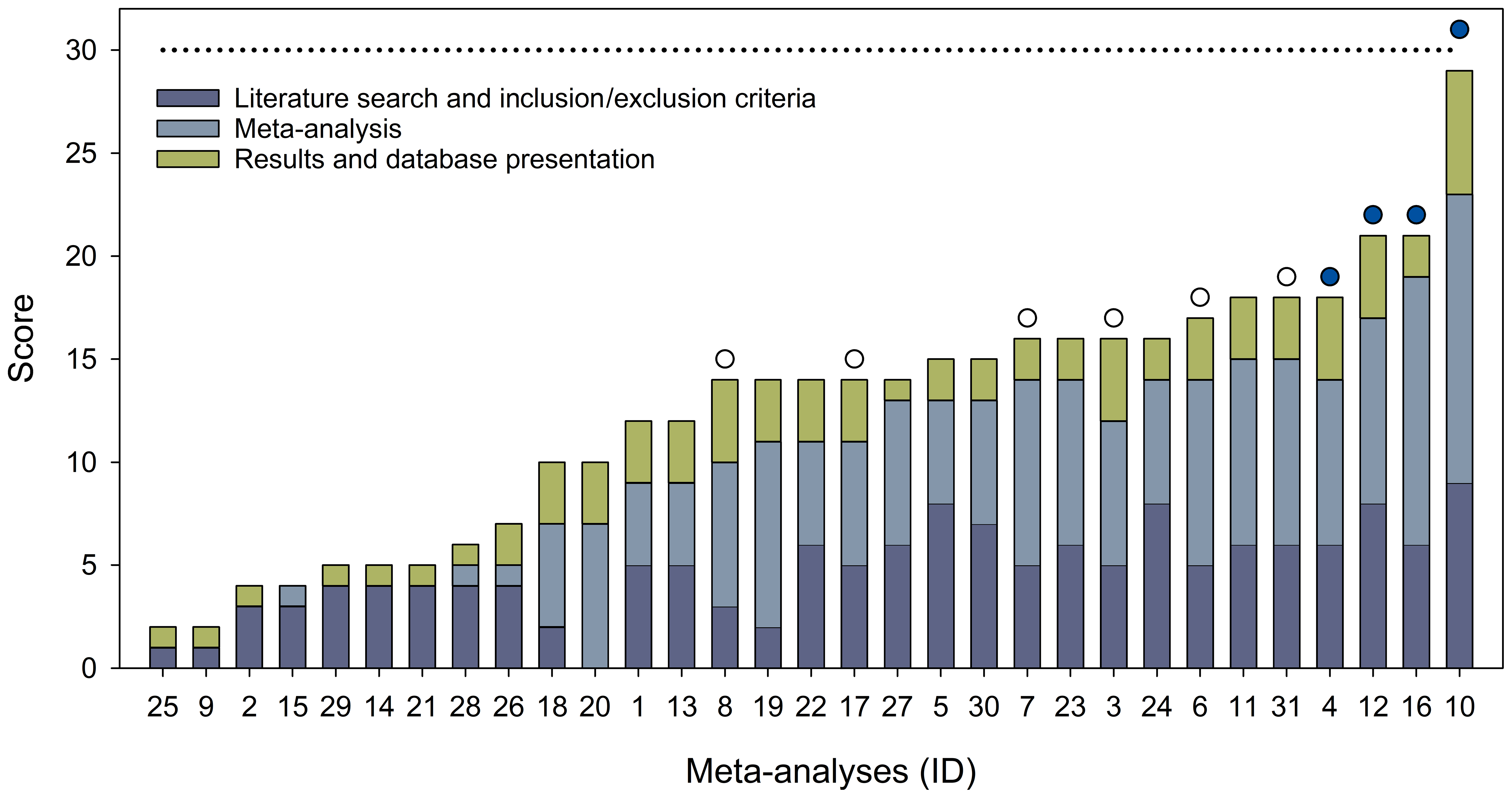 SOIL - Quality assessment of meta-analyses on soil organic carbon18 dezembro 2024
SOIL - Quality assessment of meta-analyses on soil organic carbon18 dezembro 2024 -
Analyses - Escritório de Arquit. & Eng.18 dezembro 2024
-
The Difference Between Bivariate & Multivariate Analyses18 dezembro 2024
-
 Big brand names analyses APAC nutra trends for year 202318 dezembro 2024
Big brand names analyses APAC nutra trends for year 202318 dezembro 2024 -
 Assessing and Avoiding Publication Bias in Meta-analyses18 dezembro 2024
Assessing and Avoiding Publication Bias in Meta-analyses18 dezembro 2024 -
 Functional analysis is one level of the analyses within Work18 dezembro 2024
Functional analysis is one level of the analyses within Work18 dezembro 2024
você pode gostar
-
 Minecraft Live 2023: DLC's, updates and mobs - everything we know - BBC Newsround18 dezembro 2024
Minecraft Live 2023: DLC's, updates and mobs - everything we know - BBC Newsround18 dezembro 2024 -
 COMO TER DINHEIRO INFINITO na NOVA ATUALIZAÇÃO 3.2.0 do SUBWAY18 dezembro 2024
COMO TER DINHEIRO INFINITO na NOVA ATUALIZAÇÃO 3.2.0 do SUBWAY18 dezembro 2024 -
Play FASHION DESIGNER CONTEST GAME (Game Girl FREE) - Y8.com - Vídeo Dailymotion18 dezembro 2024
-
 Labirinto do Medo: série da Netflix é quase terror e quase boa18 dezembro 2024
Labirinto do Medo: série da Netflix é quase terror e quase boa18 dezembro 2024 -
 ENTRADAS: ESPÓLIO DE ANTÓNIO SIMÕES MONTEIRO – EPHEMERA – Biblioteca e arquivo de José Pacheco Pereira18 dezembro 2024
ENTRADAS: ESPÓLIO DE ANTÓNIO SIMÕES MONTEIRO – EPHEMERA – Biblioteca e arquivo de José Pacheco Pereira18 dezembro 2024 -
 Relógio De Pulso Digital Vector Design Plano De Ilustração PNG18 dezembro 2024
Relógio De Pulso Digital Vector Design Plano De Ilustração PNG18 dezembro 2024 -
Pokémon Temporada 25 - assista todos episódios online streaming18 dezembro 2024
-
 Iskjerne Bay · The Multiverse · Roleplay on RPG18 dezembro 2024
Iskjerne Bay · The Multiverse · Roleplay on RPG18 dezembro 2024 -
 Invincible (Five album) - Wikiwand18 dezembro 2024
Invincible (Five album) - Wikiwand18 dezembro 2024 -
 Cat Mario Game Level 1-6 Full Walkthrough18 dezembro 2024
Cat Mario Game Level 1-6 Full Walkthrough18 dezembro 2024


