What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 22 fevereiro 2025

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
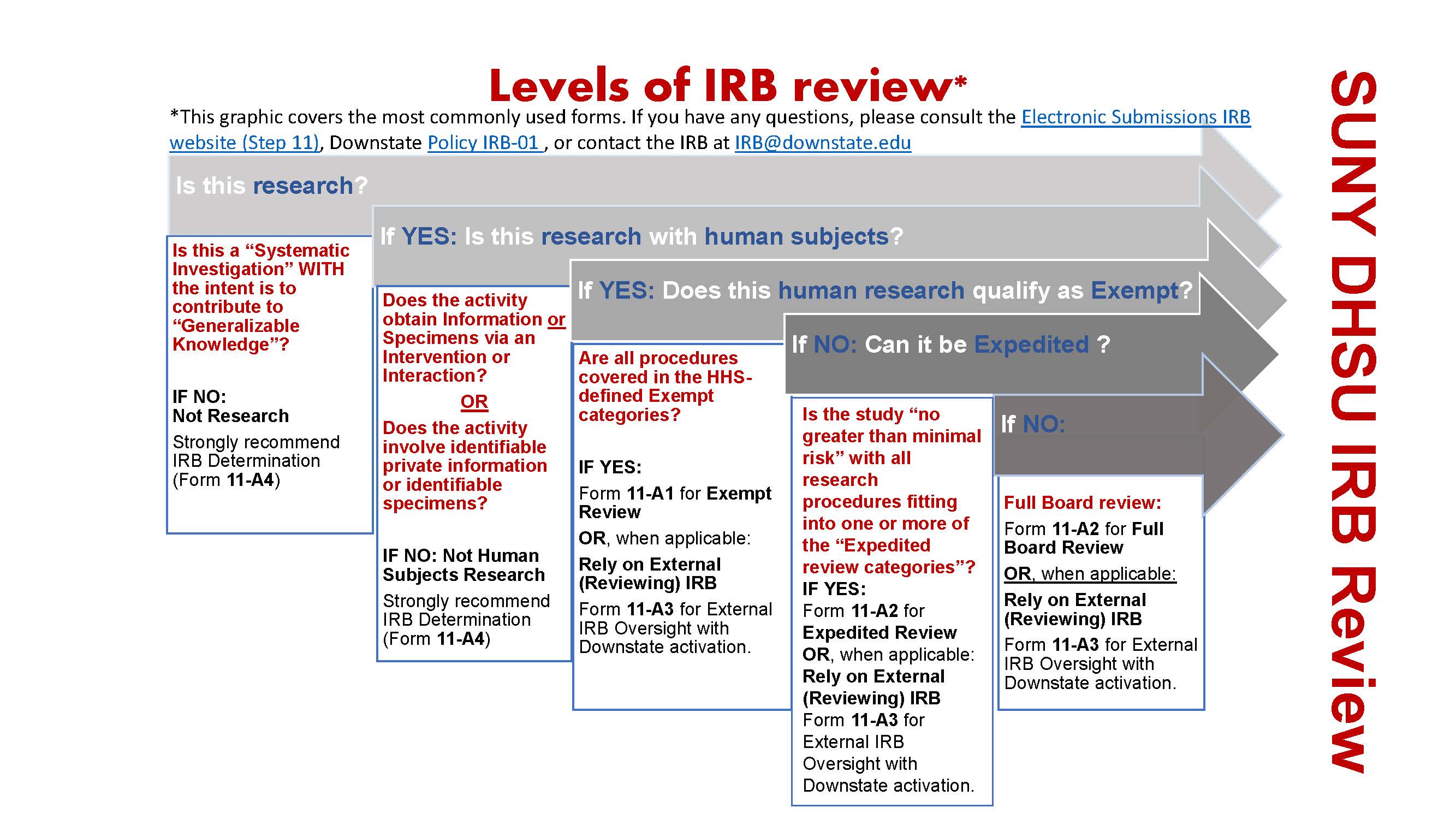
Electronic Submissions, Institutional Review Board, Office of Research Administration
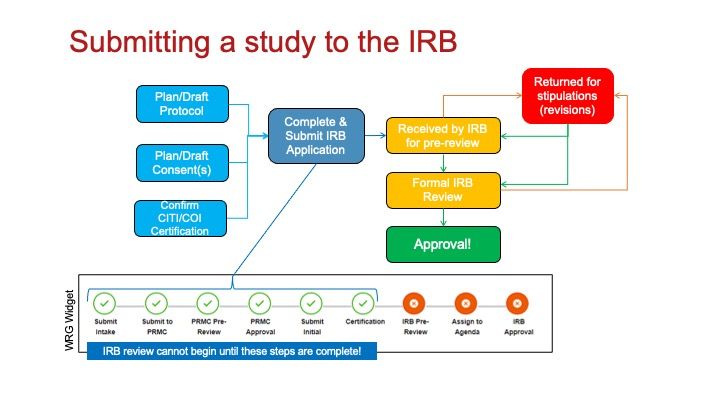
IRB Review Process Human Research Protections
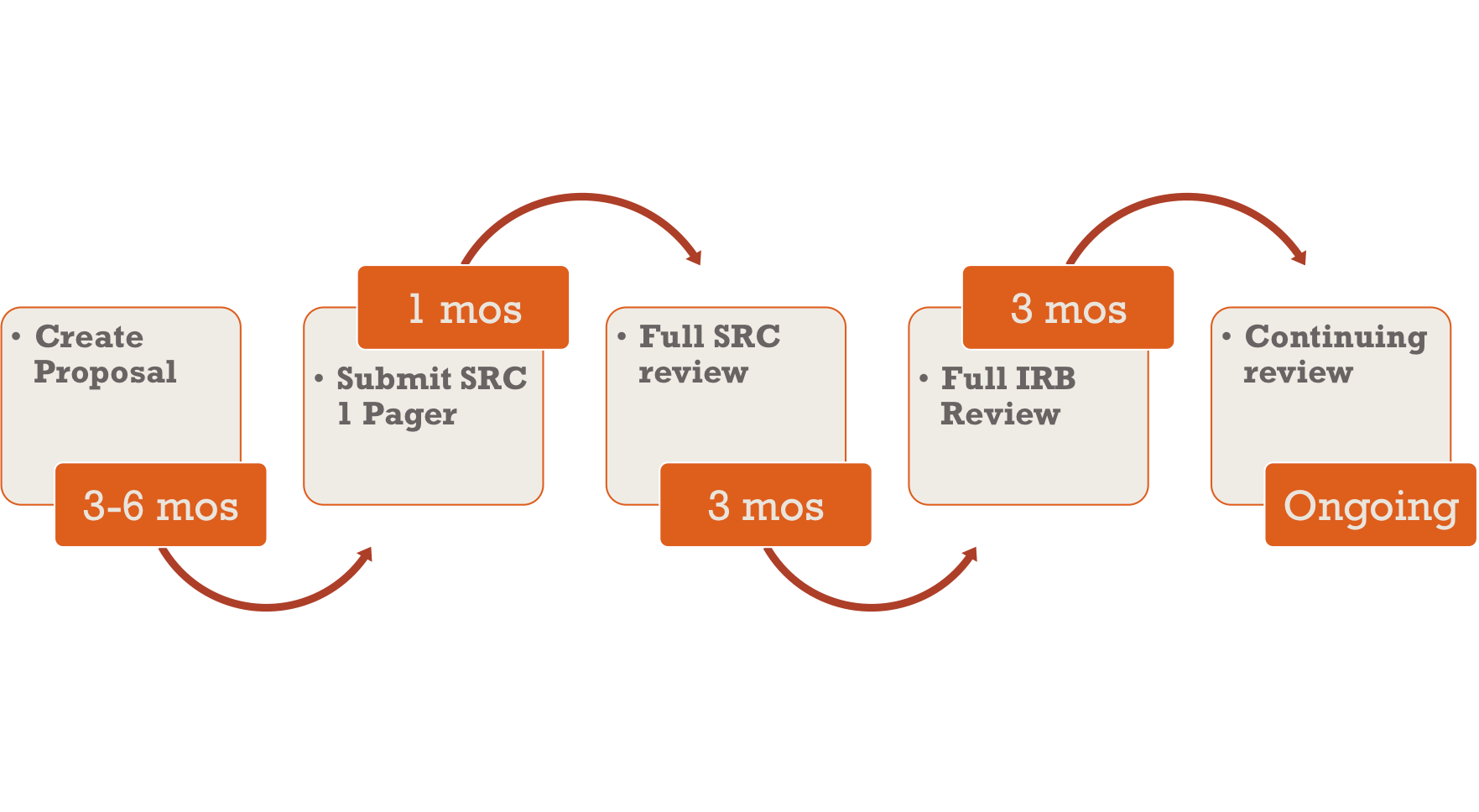
Scientific Review and IRB Submissions - National University of Natural Medicine
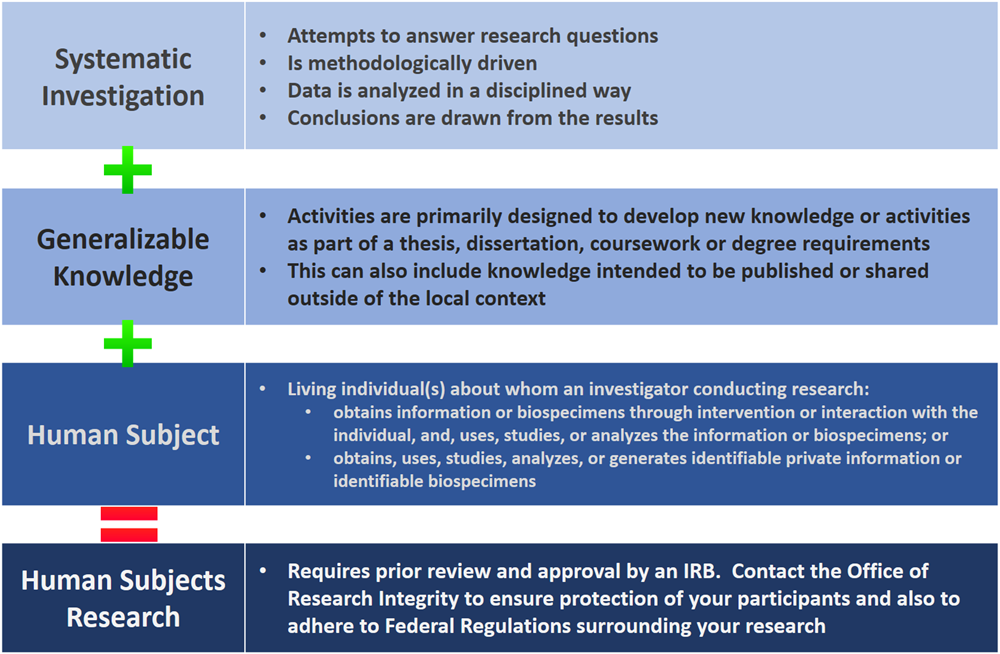
Frequently Asked Questions - Institutional Review Board (IRB)

Human Subject Participation - Office of Research Integrity
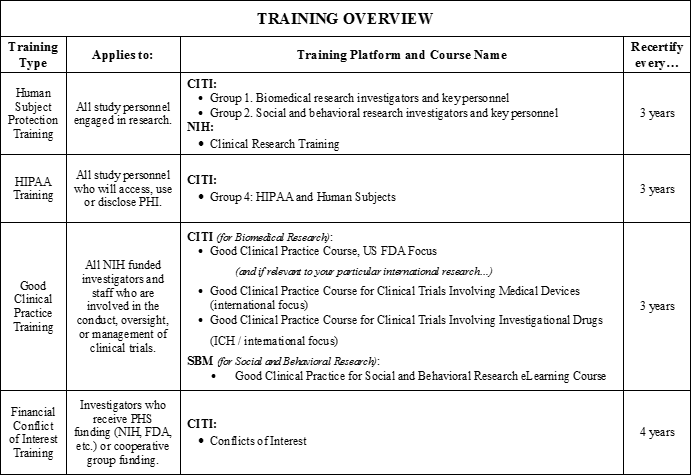
IRB 101, Office of Research Oversight/Regulatory Affairs
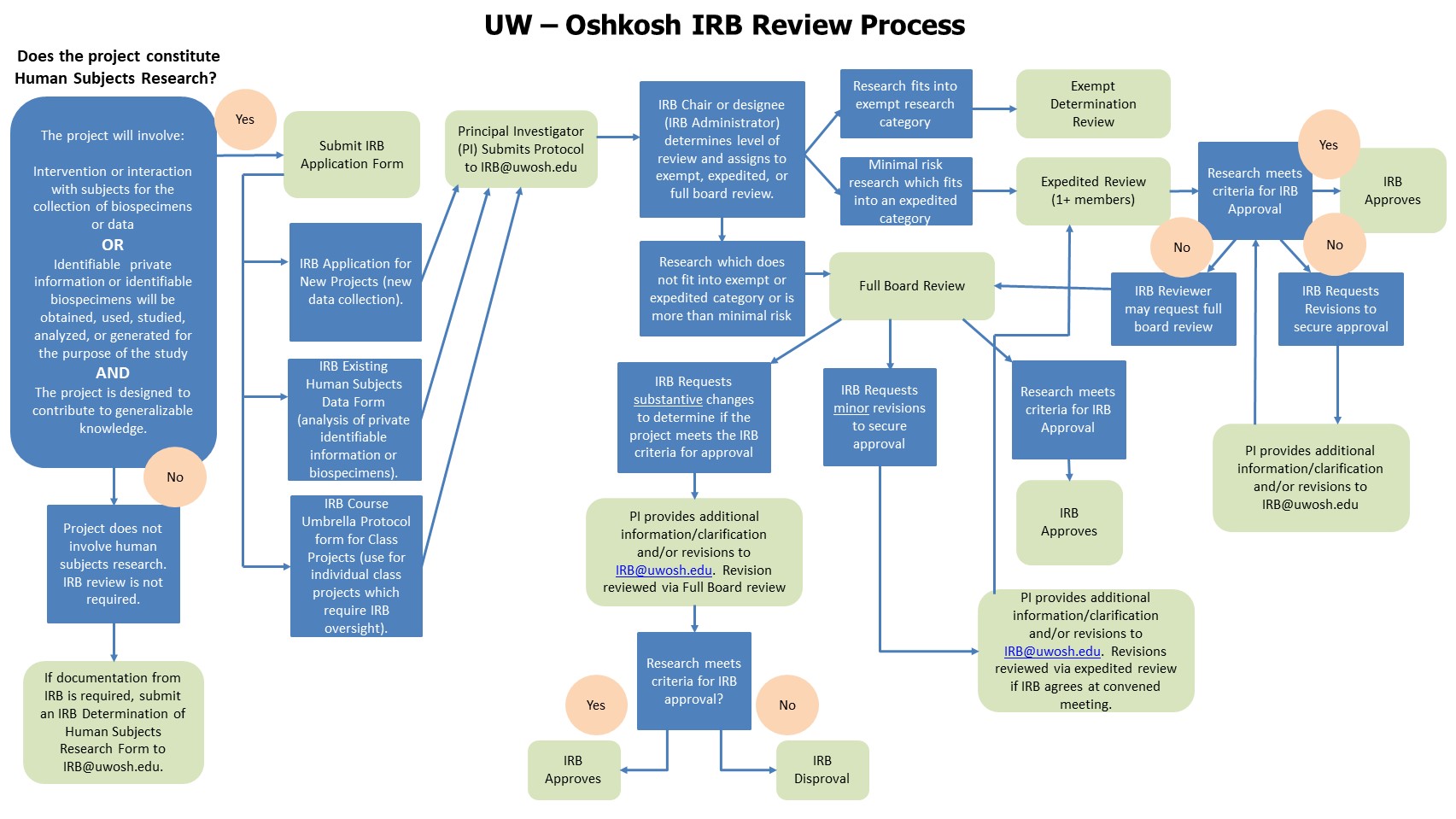
IRB Process - Office of Sponsored Programs University of Wisconsin Oshkosh
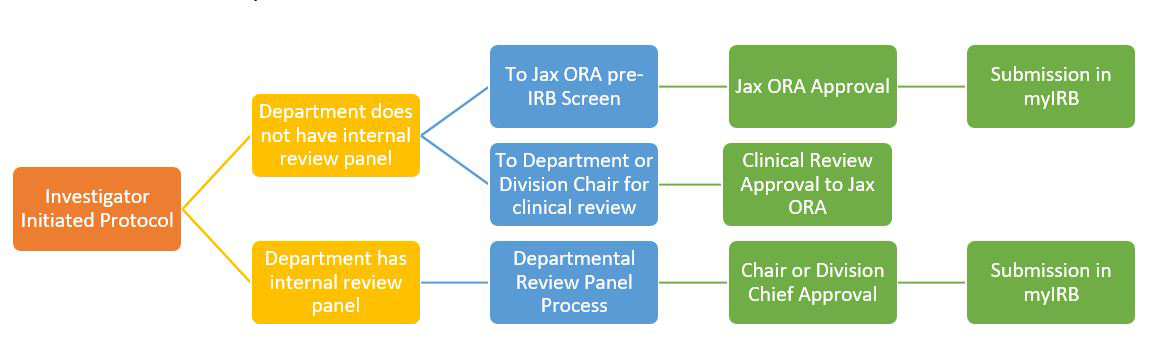
Institutional Review Board Resources, College of Medicine – Jacksonville

IRB: Institutional Review Board

Activities Requiring IRB Review - Office of Research Support and Compliance
/research.png?n=4093)
Institutional Review Board Governors State University
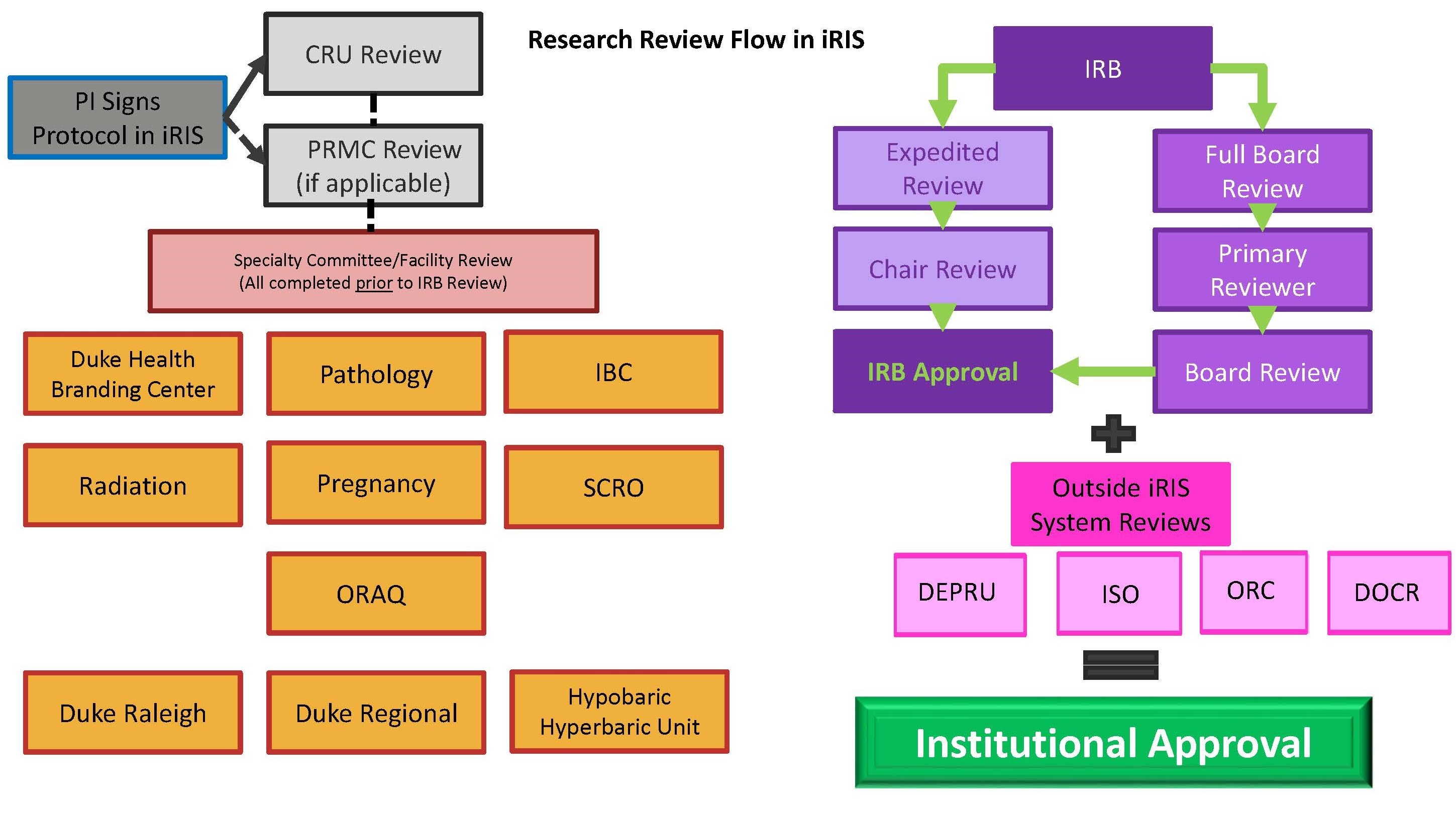
IRB Process Duke Health Institutional Review Board
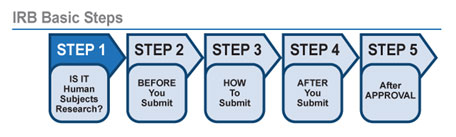
HUMAN SUBJECTS For Researchers U.S. DOE Office of Science (SC)

What Does the IRB Review?, Research
Recomendado para você
-
IRBSL - Chess Club22 fevereiro 2025
-
 BoredKoalas Funny St. Patrick's Day Pillows Irish Irish You were Naked Pun Leprechaun Funny St Patricks Day Throw Pillow, 16x16, Multicolor22 fevereiro 2025
BoredKoalas Funny St. Patrick's Day Pillows Irish Irish You were Naked Pun Leprechaun Funny St Patricks Day Throw Pillow, 16x16, Multicolor22 fevereiro 2025 -
IRBSL - ♥poussin♥☺Sidi Lakhdar22 fevereiro 2025
-
 QIDIAN Calcomanía de aleación 3D para parachoques delantero de coche, insignia para Cooper S ONE R55 R56 F54 F55 F56 F57 F60 R60 Clubman Hatchback22 fevereiro 2025
QIDIAN Calcomanía de aleación 3D para parachoques delantero de coche, insignia para Cooper S ONE R55 R56 F54 F55 F56 F57 F60 R60 Clubman Hatchback22 fevereiro 2025 -
 Casa com 4 dormitórios à venda, 141 m² por R$ 210.000,00 - S22 fevereiro 2025
Casa com 4 dormitórios à venda, 141 m² por R$ 210.000,00 - S22 fevereiro 2025 -
Joudia BOUJDAINI on LinkedIn: #railway #sustainable #transportation #africa22 fevereiro 2025
-
 Golden Sixties (1979, Vinyl) - Discogs22 fevereiro 2025
Golden Sixties (1979, Vinyl) - Discogs22 fevereiro 2025 -
 Catalog - IRB Advisors, Inc.22 fevereiro 2025
Catalog - IRB Advisors, Inc.22 fevereiro 2025 -
 انهزام يرهن الأمل - La Meskiana22 fevereiro 2025
انهزام يرهن الأمل - La Meskiana22 fevereiro 2025 -
 I found an Old Meta Team (Old Image) : r/CookieRunKingdoms22 fevereiro 2025
I found an Old Meta Team (Old Image) : r/CookieRunKingdoms22 fevereiro 2025
você pode gostar
-
 Jogo Minecraft Playstation 3 Edition - PS3 - Gabanna Games & Comics22 fevereiro 2025
Jogo Minecraft Playstation 3 Edition - PS3 - Gabanna Games & Comics22 fevereiro 2025 -
 4 Ways to Overcome Why Roblox Can't Login22 fevereiro 2025
4 Ways to Overcome Why Roblox Can't Login22 fevereiro 2025 -
 Jogo Quebra Cabeça Lógica Para Crianças Encontre Objetos22 fevereiro 2025
Jogo Quebra Cabeça Lógica Para Crianças Encontre Objetos22 fevereiro 2025 -
Mini Volante Controle PS5 Playstation Jogos De Corrida BRANCO Gamepad Controlador Alça Envio Imediato Pronta Entrega22 fevereiro 2025
-
 PlayStation Plus Free Games: What Should Sony Offer?22 fevereiro 2025
PlayStation Plus Free Games: What Should Sony Offer?22 fevereiro 2025 -
 Women's World Chess Championship 2023 - Wikiwand22 fevereiro 2025
Women's World Chess Championship 2023 - Wikiwand22 fevereiro 2025 -
 5 Best Snake Games for iPhone in 202322 fevereiro 2025
5 Best Snake Games for iPhone in 202322 fevereiro 2025 -
 Speech: John D. Rockefeller Jr. Sets Forth His Family's Creed22 fevereiro 2025
Speech: John D. Rockefeller Jr. Sets Forth His Family's Creed22 fevereiro 2025 -
 VicTycoon Art - AVATAR - Canal Guii22 fevereiro 2025
VicTycoon Art - AVATAR - Canal Guii22 fevereiro 2025 -
 SAMURAI MAIDEN on Steam22 fevereiro 2025
SAMURAI MAIDEN on Steam22 fevereiro 2025


