What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 18 dezembro 2024

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

Complete guide to computer system validation in 2023

Computer System Validation - JAF Consulting
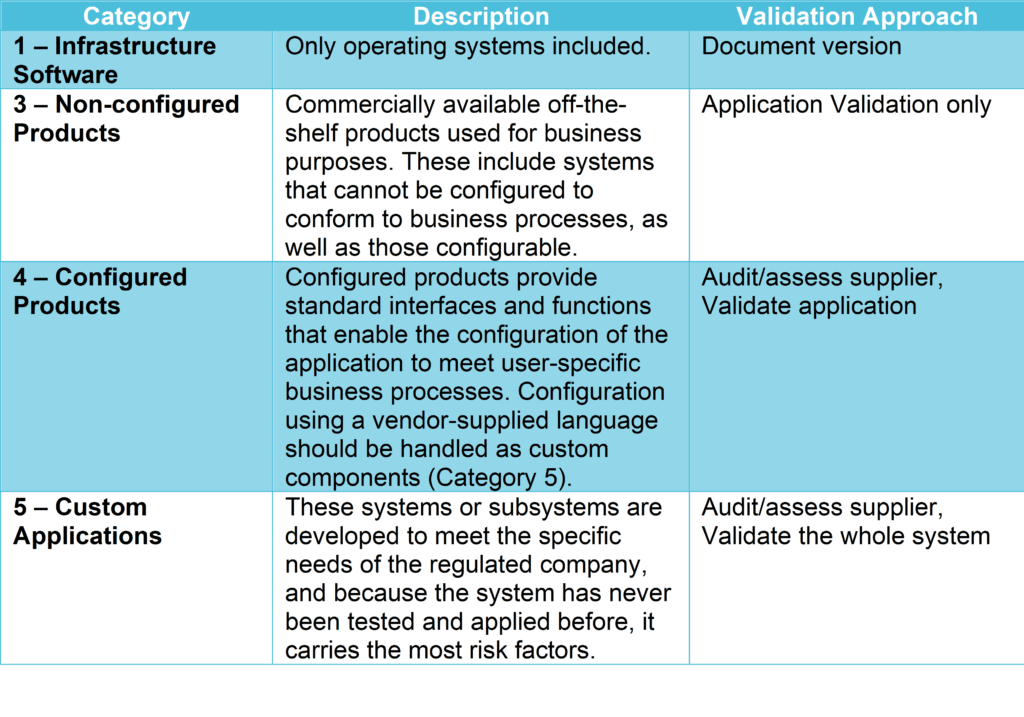
Risk-Based Computerized System Validation (CSV) and Computer Software Assurance (CSA) - Old Wine in a New Bottle? - Kvalito

Computer System Validation (CSV) in Life Sciences Part 1: Introduction to CSV - Verista

Types of Validation in the Pharmaceutical Industry
PDF) Computerized Systems Validation (CSV) in Biopharmaceutical Industries
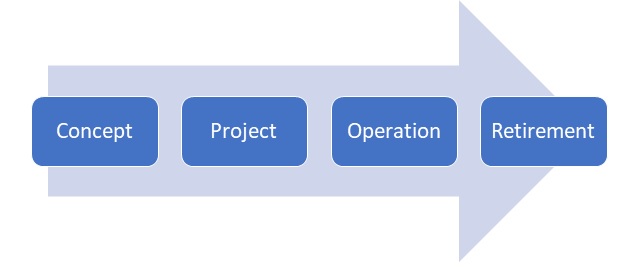
CSV Life Cycle - Gsap

What is Computer System Validation?

Importance of computer system validation (CSV) in Pharmaceutical Industry
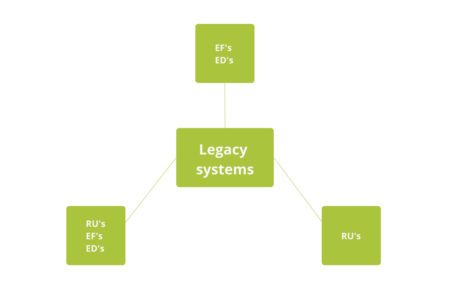
A Complete Guide to Computer System Validation (CSV): What is it and why do we need it?

Computer System Validation in Pharmaceutical Industry

Pharmaceutical Computer Validation Certificate Program • NACPT
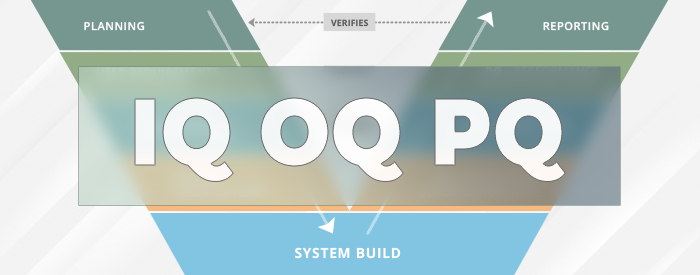
What is IQ OQ PQ in Software Validation?
Data Integrity in the Pharmaceutical Industry
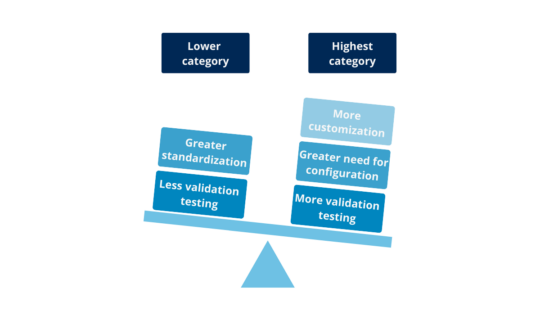
A Complete Guide to Computer System Validation (CSV): What is it and why do we need it?
Recomendado para você
-
 DataMap PO SmartView Coupa App Marketplace18 dezembro 2024
DataMap PO SmartView Coupa App Marketplace18 dezembro 2024 -
💙 VEM SER VICENTINO! 💙 - Colégio São Vicente de Paulo18 dezembro 2024
-
 Save on Columbus Salame Sopressata Chub Order Online Delivery18 dezembro 2024
Save on Columbus Salame Sopressata Chub Order Online Delivery18 dezembro 2024 -
 CSV1A CYCLE STOP VALVE – Cycle Stop Valves, Inc18 dezembro 2024
CSV1A CYCLE STOP VALVE – Cycle Stop Valves, Inc18 dezembro 2024 -
import csv file - SAS Support Communities18 dezembro 2024
-
 Creating a csv file in other format than comma for master or18 dezembro 2024
Creating a csv file in other format than comma for master or18 dezembro 2024 -
 California School Personnel Commissioners Association18 dezembro 2024
California School Personnel Commissioners Association18 dezembro 2024 -
 2012 Audi A4 2.0 TDI S LINE 2.0 Diesel Manual - £8500 - PMA Cars18 dezembro 2024
2012 Audi A4 2.0 TDI S LINE 2.0 Diesel Manual - £8500 - PMA Cars18 dezembro 2024 -
 2012 Audi A4 2.0 TDI S LINE 2.0 Diesel Manual - £8500 - PMA Cars - Cars NI18 dezembro 2024
2012 Audi A4 2.0 TDI S LINE 2.0 Diesel Manual - £8500 - PMA Cars - Cars NI18 dezembro 2024 -
 2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars - Cars NI18 dezembro 2024
2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars - Cars NI18 dezembro 2024
você pode gostar
-
 SNK vs. Capcom series, Street Fighter Wiki18 dezembro 2024
SNK vs. Capcom series, Street Fighter Wiki18 dezembro 2024 -
scary teacher 3d. fun in the sun. New updated level. scary teacher. prank with miss t. - video Dailymotion18 dezembro 2024
-
 OC) Weapon wielding adult green dragon : r/Pathfinder2e18 dezembro 2024
OC) Weapon wielding adult green dragon : r/Pathfinder2e18 dezembro 2024 -
Shu Kurenai - Shu Kurenai added a new photo.18 dezembro 2024
-
 Moses Ingram responds to racism from 'Star Wars' fans - Upworthy18 dezembro 2024
Moses Ingram responds to racism from 'Star Wars' fans - Upworthy18 dezembro 2024 -
 Young gru meme by DracoAwesomeness on DeviantArt18 dezembro 2024
Young gru meme by DracoAwesomeness on DeviantArt18 dezembro 2024 -
Maraculois ladybug and cat noire theme song #ladybug #ladybugandcatnoi, miraculous song english18 dezembro 2024
-
 Aos 46 anos, Ivan Moré fala sobre namorada de 23: 'Mãe dela é mais18 dezembro 2024
Aos 46 anos, Ivan Moré fala sobre namorada de 23: 'Mãe dela é mais18 dezembro 2024 -
 Vestido Festa Princesa Sofia Luxo Mod.618 dezembro 2024
Vestido Festa Princesa Sofia Luxo Mod.618 dezembro 2024 -
 Hanseníase: o que é, tratamento, tipos e sintomas - Brasil Escola18 dezembro 2024
Hanseníase: o que é, tratamento, tipos e sintomas - Brasil Escola18 dezembro 2024