Assembly of recombinant tau into filaments identical to those of
Por um escritor misterioso
Last updated 19 janeiro 2025
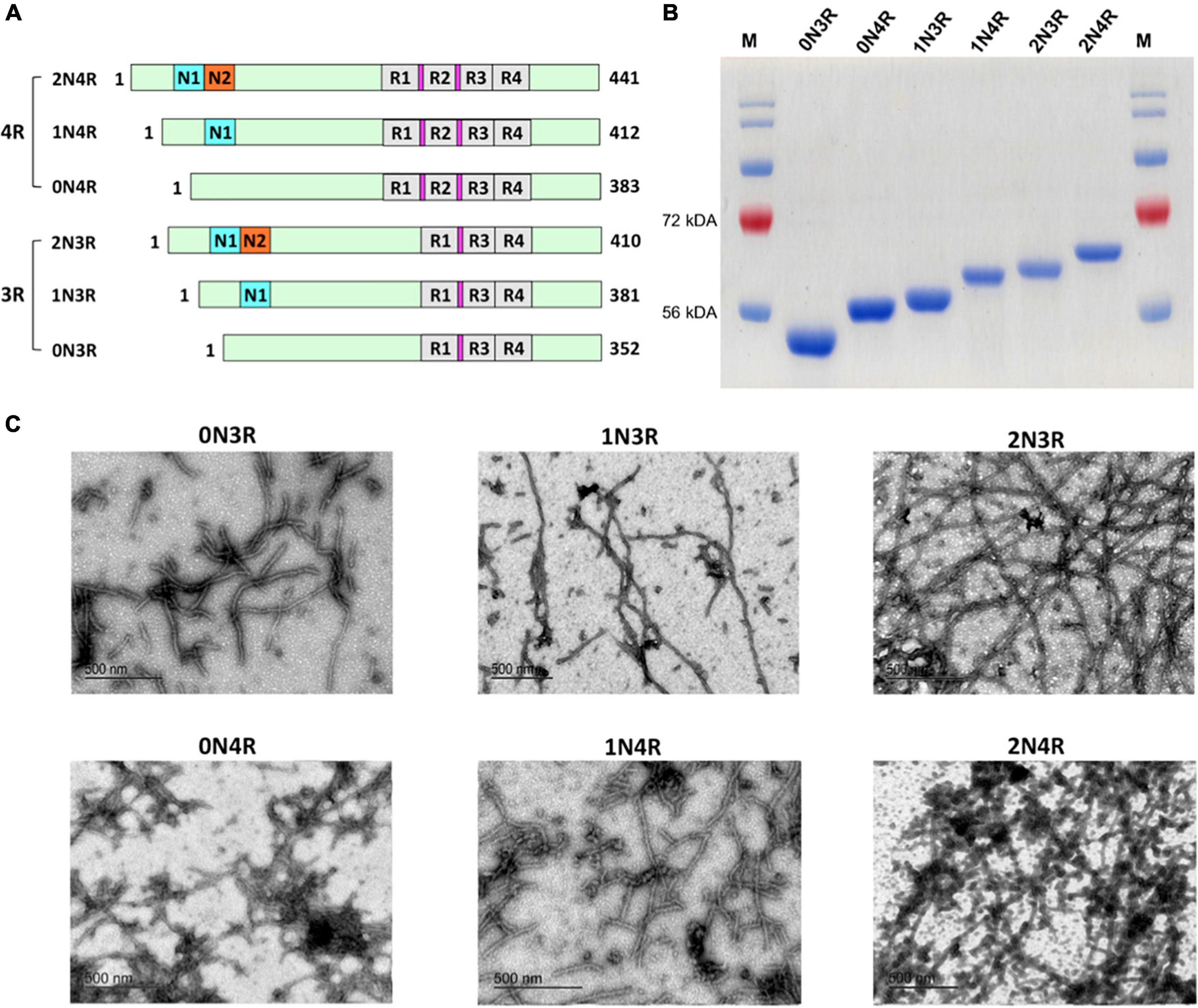
Many neurodegenerative diseases, including Alzheimer’s disease, the most common form of dementia, are characterised by knotted clumps of a protein called tau. In these diseases, tau misfolds, stacks together and forms abnormal filaments, which have a structured core and fuzzy coat. These sticky, misfolded proteins are thought to be toxic to brain cells, the loss of which ultimately causes problems with how people move, think, feel or behave. Reconstructing the shape of tau filaments using an atomic-level imaging technique called electron cryo-microscopy, or cryo-EM, researchers have found distinct types of tau filaments present in certain diseases. In Alzheimer’s disease, for example, a mixture of paired helical and straight filaments is found. Different tau filaments are seen again in chronic traumatic encephalopathy (CTE), a condition associated with repetitive brain trauma. It remains unclear, however, how tau folds into these distinct shapes and under what conditions it forms certain types of filaments. The role that distinct tau folds play in different diseases is also poorly understood. This is largely because researchers making tau proteins in the lab have yet to replicate the exact structure of tau filaments found in diseased brain tissue. Lövestam et al. describe the conditions for making tau filaments in the lab identical to those isolated from the brains of people who died from Alzheimer’s disease and CTE. Lövestam et al. instructed bacteria to make tau protein, optimised filament assembly conditions, including shaking time and speed, and found that bona fide filaments formed from shortened versions of tau. On cryo-EM imaging, the lab-produced filaments had the same left-handed twist and helical symmetry as filaments characteristic of Alzheimer’s disease. Adding salts, however, changed the shape of tau filaments. In the presence of sodium chloride, otherwise known as kitchen salt, tau formed filaments with a filled cavity at the core, identical to tau filaments observed in CTE. Again, this structure was confirmed on cryo-EM imaging. Being able to make tau filaments identical to those found in human tauopathies will allow scientists to study how these filaments form and elucidate what role they play in disease. Ultimately, a better understanding of tau filament formation could lead to improved diagnostics and treatments for neurodegenerative diseases involving tau.
Laboratory-based methods are presented that produce filamentous tau aggregates with the same structures as those observed in neurodegenerative disease.
Laboratory-based methods are presented that produce filamentous tau aggregates with the same structures as those observed in neurodegenerative disease.

Conformation Determines the Seeding Potencies of Native and Recombinant Tau Aggregates - ScienceDirect
Frontiers Selective Detection of Misfolded Tau From Postmortem Alzheimer's Disease Brains

Cryo-EM structure of RNA-induced tau fibrils reveals a small C-terminal core that may nucleate fibril formation

Cryo-EM structure of RNA-induced tau fibrils reveals a small C-terminal core that may nucleate fibril formation

Cryo-EM structure of RNA-induced tau fibrils reveals a small C-terminal core that may nucleate fibril formation
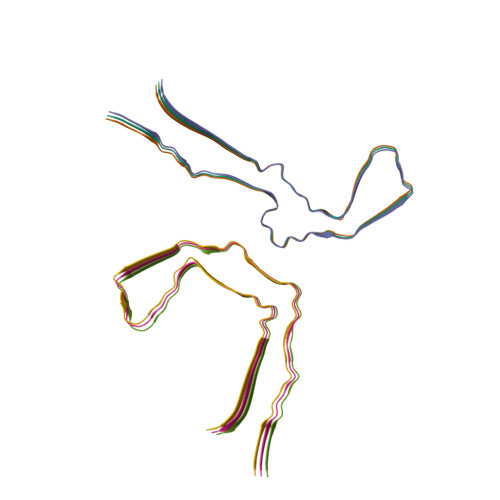
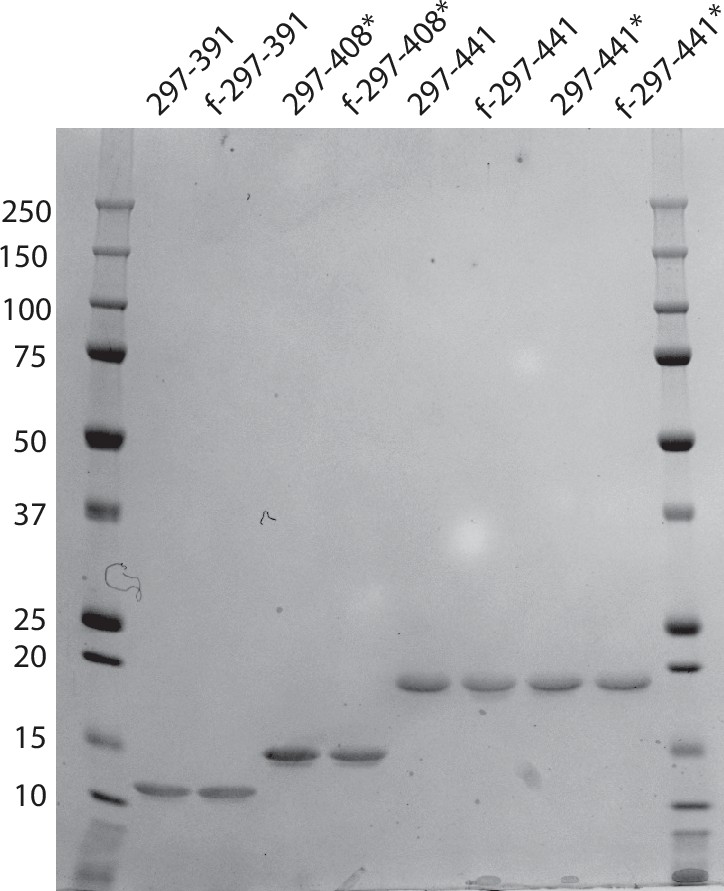
RCSB PDB - 7QK1: In vitro assembled 297-394 tau filaments in PBS (35d)
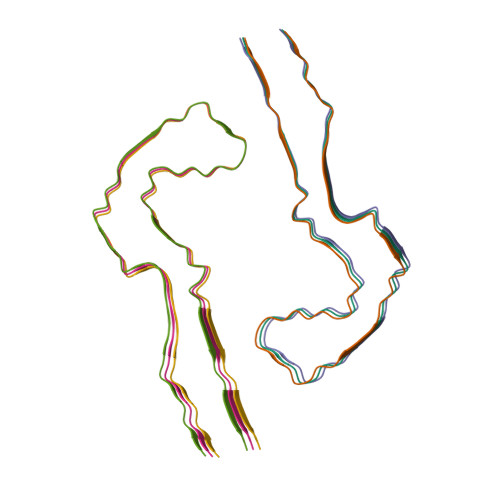
RCSB PDB - 7QL3: in vitro assembled 266/297 - 391 tau filaments with NaCl (8b)

Subtle change of fibrillation condition leads to substantial alteration of recombinant Tau fibril structure - ScienceDirect
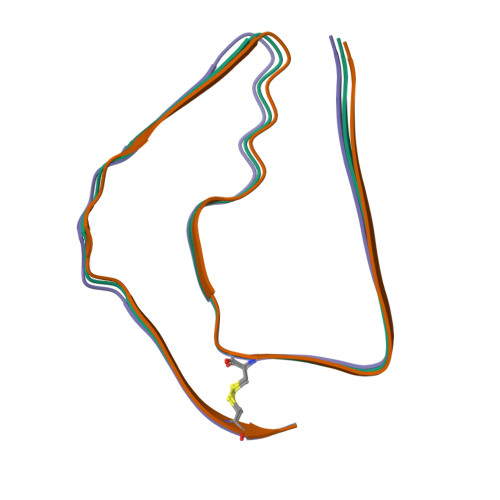
RCSB PDB - 7QL2: In vitro assembled 244-391 tau filaments with Na2P2O7 (20a)
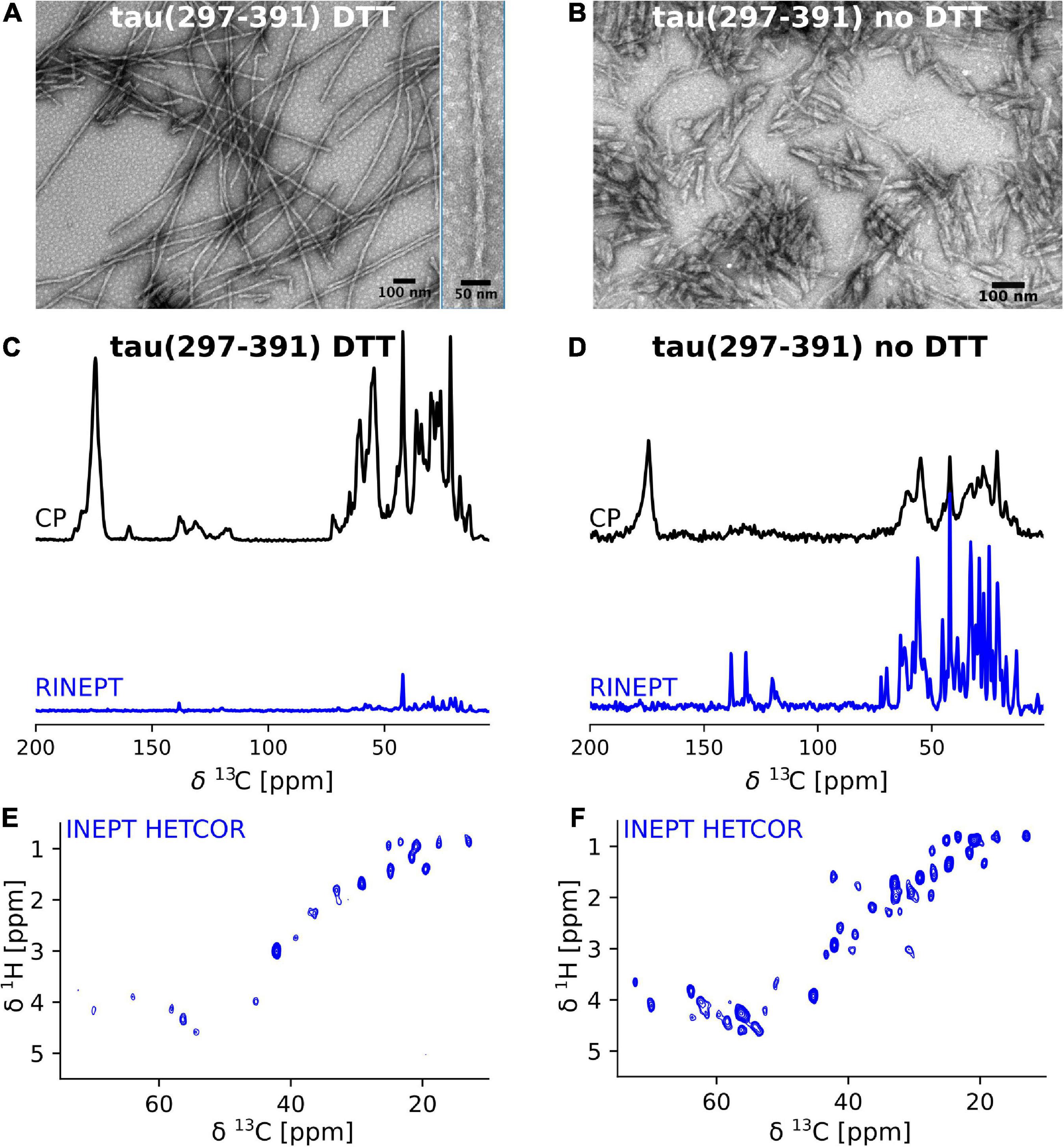
Frontiers Solid-state NMR of paired helical filaments formed by the core tau fragment tau(297-391)
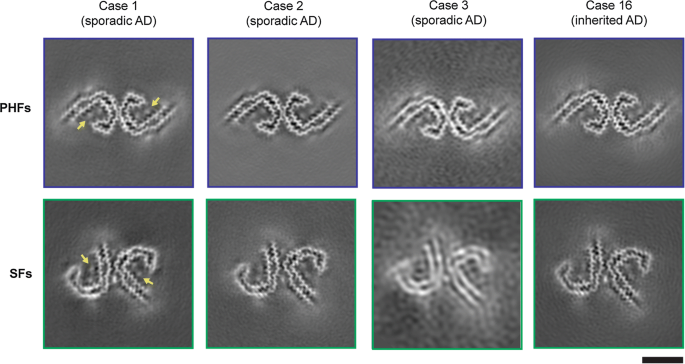
Tau filaments from multiple cases of sporadic and inherited Alzheimer's disease adopt a common fold
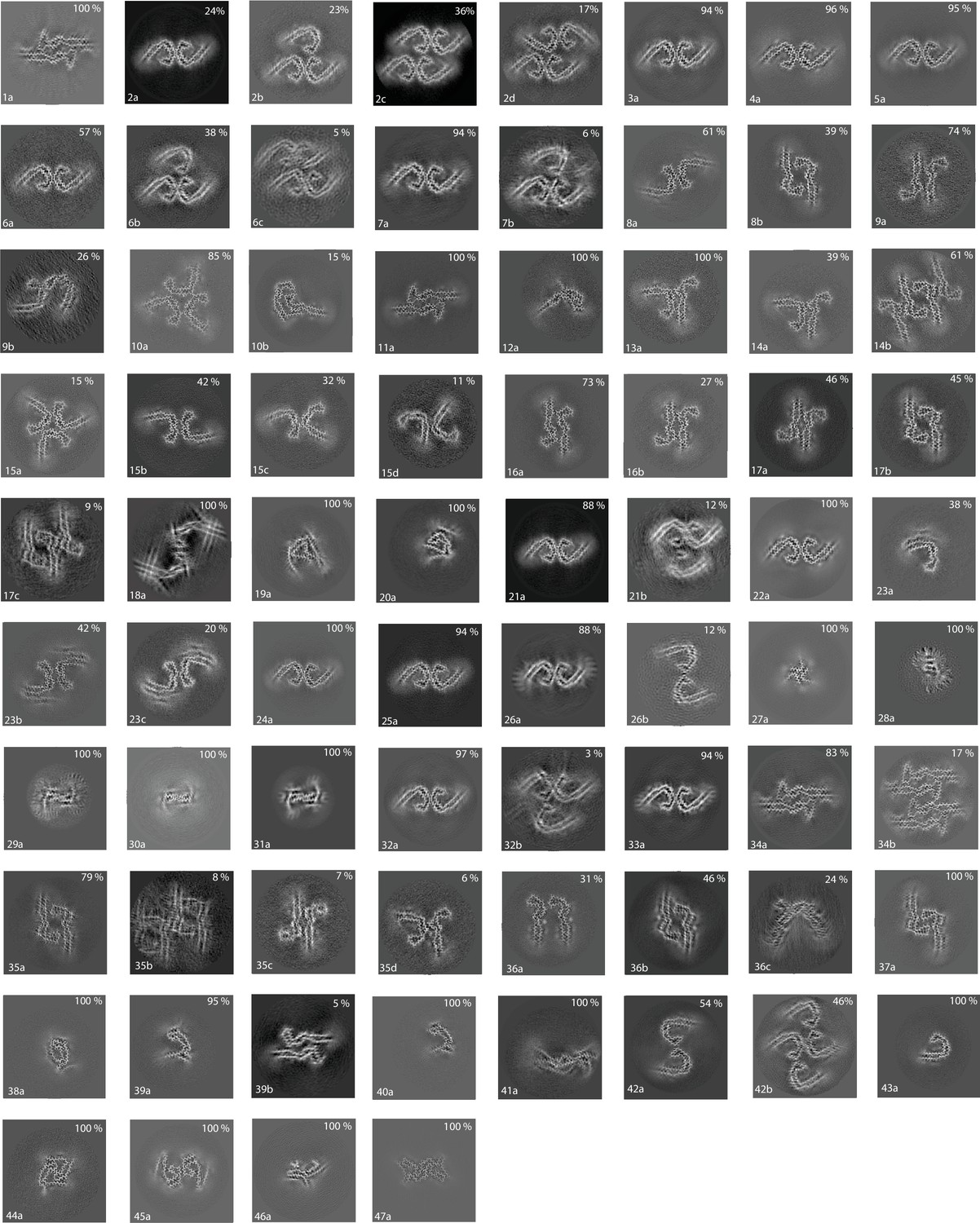
Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy
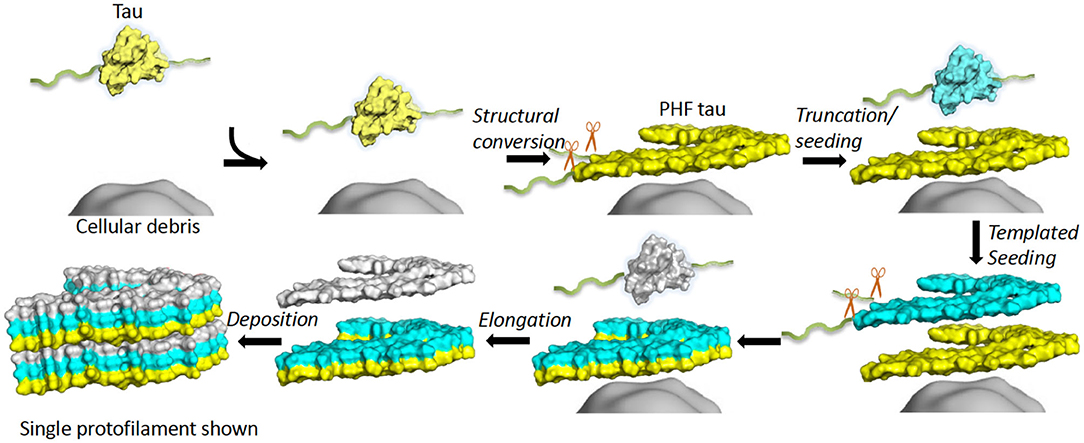
Frontiers Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target
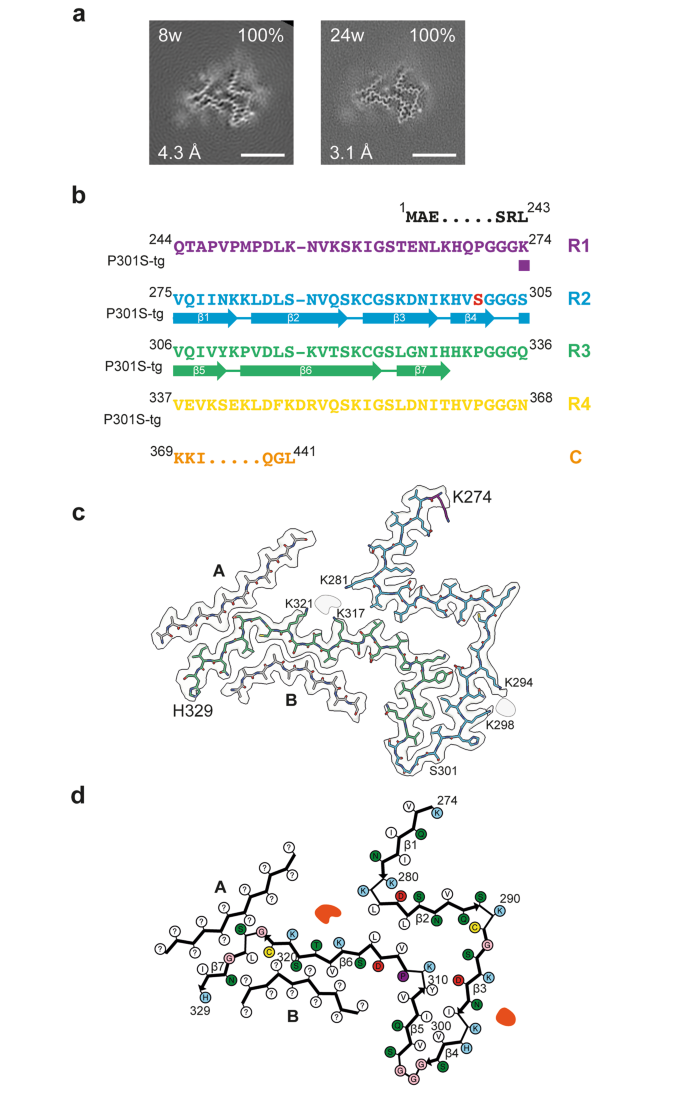
Cryo-EM structures of tau filaments from the brains of mice transgenic for human mutant P301S Tau, Acta Neuropathologica Communications
Recomendado para você
-
Brain Test Game Level 287 to 304 Walkthrough, Brain Test Game Level 287 to 304, Brain Test Level 287, 288, 289, 290, 291, 292, 293, 294, 295, 296, 297, 298, 299, 300, 301, 302, 303, 304 Walkthrough, By Dangamevideos19 janeiro 2025
-
 حل Brain Test من المرحلة 280 إلى المرحلة 30019 janeiro 2025
حل Brain Test من المرحلة 280 إلى المرحلة 30019 janeiro 2025 -
 brain test nível 29719 janeiro 2025
brain test nível 29719 janeiro 2025 -
 Brain test 3 level 29719 janeiro 2025
Brain test 3 level 29719 janeiro 2025 -
 IBM WebSphere MQ V5.3 Solution Development Visit: - ppt download19 janeiro 2025
IBM WebSphere MQ V5.3 Solution Development Visit: - ppt download19 janeiro 2025 -
 Frontiers Diurnal Variations in Neural Activity of Healthy Human19 janeiro 2025
Frontiers Diurnal Variations in Neural Activity of Healthy Human19 janeiro 2025 -
 NEURO EMERGENCIES - Winter 2016 - day 6 Flashcards19 janeiro 2025
NEURO EMERGENCIES - Winter 2016 - day 6 Flashcards19 janeiro 2025 -
 Draw Toilet : Squid Game APK for Android Download19 janeiro 2025
Draw Toilet : Squid Game APK for Android Download19 janeiro 2025 -
 Premium Vector Old style layout design template19 janeiro 2025
Premium Vector Old style layout design template19 janeiro 2025 -
 COVID-19 in pregnancy: implications for fetal brain development19 janeiro 2025
COVID-19 in pregnancy: implications for fetal brain development19 janeiro 2025
você pode gostar
-
 International Chess Federation on X: Iran-born teenager Alireza Firouzja scored a phenomenal 8/9 and surpassed the 2800 mark in the live ratings. Alireza will most likely jump to number 2 in the19 janeiro 2025
International Chess Federation on X: Iran-born teenager Alireza Firouzja scored a phenomenal 8/9 and surpassed the 2800 mark in the live ratings. Alireza will most likely jump to number 2 in the19 janeiro 2025 -
 HD wallpaper: two male and one female anime characters19 janeiro 2025
HD wallpaper: two male and one female anime characters19 janeiro 2025 -
 Como fazer Vestido de Festa para Barbie19 janeiro 2025
Como fazer Vestido de Festa para Barbie19 janeiro 2025 -
 Bunny Girl, seishun buta yarou, HD phone wallpaper19 janeiro 2025
Bunny Girl, seishun buta yarou, HD phone wallpaper19 janeiro 2025 -
 Buy Nintendo Switch Vampire: The Masquerade Swansong19 janeiro 2025
Buy Nintendo Switch Vampire: The Masquerade Swansong19 janeiro 2025 -
 Code Veronica - : r/residentevil19 janeiro 2025
Code Veronica - : r/residentevil19 janeiro 2025 -
 Vivonne sauvignon 1988 – Aoneliquors19 janeiro 2025
Vivonne sauvignon 1988 – Aoneliquors19 janeiro 2025 -
 Decathlon Lands In San Francisco19 janeiro 2025
Decathlon Lands In San Francisco19 janeiro 2025 -
 LISTA DOS EPISÓDIOS FILLERS DE NARUTO SHIPPUDEN19 janeiro 2025
LISTA DOS EPISÓDIOS FILLERS DE NARUTO SHIPPUDEN19 janeiro 2025 -
 Conhecemos o set de gravações da FOFA série de comédia Amsterdam, da HBO Max!19 janeiro 2025
Conhecemos o set de gravações da FOFA série de comédia Amsterdam, da HBO Max!19 janeiro 2025
